Research Article - Onkologia i Radioterapia ( 2022) Volume 16, Issue 1
A dosimetric comparison between three dimensional conformal radiation therapy and volumetric-modulated arc therapy for medulloblastoma craniospinal irradiation
Abdelati Nourreddine1,2, Maroua Benlemlih2*, Abdelhak Maghous3, Sanae El Mejjaoui2, Tayeb Kebdani2, Khalid Hassouni2, Hanane EL kacemi2, Noureddine Benjaafar2 and Rajaa Cherkaoui El Moursli12Department of Radiotherapy, National Institute of Oncology, Rabat, Morocco
3Department of Radiotherapy, Mohamed V Military Hospital, Rabat, Morocco
Maroua Benlemlih, Department of Radiotherapy, National Institute of Oncology, Rabat, Morocco, Email: maroua.benlemlih@gmail.com
Received: 31-Dec-2021, Manuscript No. M- 50965; Accepted: 21-Jan-2022, Pre QC No. P-50965; Editor assigned: 03-Jan-2022, Pre QC No. P-50965; Reviewed: 15-Jan-2022, QC No. Q-50965; Revised: 21-Jan-2022, Manuscript No. R-50965; Published: 26-Jan-2022
Abstract
Aims: To compare Three-Dimensional Conformal Radiation Therapy (3DCRT) and Volumetric‑Modulated Arc Therapy (VMAT) in Craniospinal Irradiation (CSI) with Posterior Fossa (PF) boost in children with Medulloblastoma (MB); dosimetry evaluation and comparison of both techniques with regard to target coverage and doses to organs at risk. Patients and Methods: Ten previously irradiated patients of MB treated with VMAT were retrieved and re‑planned with 3D-CRT technique. Dosimetric comparison was done of the two plans. Prescription dose and normal tissue constraints were identical for both plans. Statistical Analysis Used: SPSS, version 25.0, statistical software package was used. For quantitative data, Anova and Post Hoc tests were applied to calculate the difference between two means. Results: The dose homogeneity was better in VMAT (0, 07) as compared to 3D-CRT (0, 12), with statistically significant difference (P=0.043). Conformity index was also better with VMAT technique (1, 08) than 3D-CRT (1, 34) with P=0.000. VMAT plan provided reduced mean dose and V20 to almost all organ at risk evaluated with a statistically significant difference. Conclusions: VMAT technique was able to improve homogeneity and conformity index, spare high dose to normal tissues
Keywords
craniospinal irradiation, volumetric modulated arc therapy, threedimensional conformal radiotherapy, medulloblastoma, organ at risk
Introduction
Medulloblastoma (MB), which can spread through the cerebrospinal fluid, is a malignant primitive neuroectodermal tumour that originates from the Posterior Cranial Fossa (PCF). A total of 80% of medulloblastoma patients are diagnosed when they are younger than 15 years of age (median age, 5 years) [1]. The incidence of Adult Medulloblastoma (AMB) is approximately 0.5/100000 [2, 3], accounting for 0.4%-1% of adult nervous system tumours [4]. Surgery is the first treatment choice for non-metastatic MB, and all patients should be treated with Craniospinal Irradiation (CSI) postoperatively.
Craniospinal Irradiation (CSI) is integral in the definitive management of medulloblastoma. Improvements in therapy have resulted in 5-year overall survival rates in excess of 80% for average-risk medulloblastoma [5]. But long-term survivors experience a multitude of late effects, including neurocognitive decline, endocrine deficits, hearing loss, growth retardation, vasculopathies, and somatic effects. In particular, data support a clear correlation of irradiated volume, which is extensive in CSI, to risk of secondary malignancy [6, 7].
The traditional Craniospinal Irradiation (CSI) technique typically treats the Central Nervous System (CNS) using classic 3D Conformal Radiation Therapy (3DCRT) with opposed lateral fields to treat the brain and posterior fields to treat the spine. This technique does not spare any organs and causes significant acute and late morbidities. Also, Matched junctions between fields result in inhomogeneous dose regions and require feathering, which increases the complexity of planning and delivery.
Recently, Volumetric Modulated Arc Therapy (VMAT) has been evaluated. It uses single or multiple arcs to deliver highly conformal doses to the Planning Target Volume (PTV).
This report compares VMAT with 3D-CRT in a treatment planning study of 10 cases of childhood medulloblastoma to evaluate differences in conformity, homogeneity indices and normal tissue sparing.
Patients and Methods
Patients
During a period between July 2018 and October 2021, 22 medullolastoma were treated at radiotherapy department with VMAT technique. Among these patients, we selected 10 children with standard-risk medulloblastoma that were treated in a supine position with two isocenters.
Simulation in supine position with thermoplastic mask and a vacuum cushion. Computed Tomography (CT) images were acquired using CT scanner with 3-mm slice intervals from the vertex to 10 cm below the S5 vertebra.
Each of these previously irradiated patients of MB was retrieved and re-planned with both 3DCRT techniques for dosimetric comparison.
Delineation of the target volume and organs at risk
Clinical Target Volumes (CTV) and OAR were demarcated on axial CT images. The craniospinal CTV encompassed the brain, the spinal cord, and the covering meninges. The lateral border of the CTV and the caudal extent of the thecal sac were identified from a T2-weighted magnetic resonance imaging scan. For the Planning Target Volume (PTV), the CTV was expanded uniformly by a margin of 5 mm for the brain and 10 mm for the spinal cord. The boost CTV included the entire posterior cerebral fossa. OARs included the brain, eyes, lenses, optical nerves, optic chiasm, thyroid, pituitary, heart, lungs, liver, oesophagus, kidneys, testis or ovaries, uterus, and breasts.
Treatment planning
The CSI dose for all patients was 36 Gy (20 fractions of 1.8 Gy), followed by a posterior fossa boost to 54 Gy using an additional 18 fractions of 1.8 Gy.
3D-CRT plan uses “integrated gap feathering”: the first two opposed lateral beams for cranial irradiation with collimator rotation of 7º -10º to match the divergence of the posterior beam for the spinal irradiation, and the second set of the two opposed lateral beams with the lower cervical border shifted by 1 cm, 5 cm, to change the level of the junction with the spinal beam in addition to a posterior beam for the spinal field.
VMAT-based treatment plans were generated for each patient. It used three coplanar arcs: one complete arc (360º) to cover the superior portion of the TV (brain and upper portion of the spinal cord) and two partial arcs (30º each), with opposite direction from 180º position to cover the inferior portion of TV (the rest of the spinal cord).
Dose calculations used inverse planning optimization (Monaco treatment planning system version 5.11.02) and a Monte Carlo algorithm (Elekta AB, Sweden). Plan optimization used biological cost function: equivalent uniform dose for PTVs and serial/parallel cost functions for OAR. Craniospinal treatment plans used 6MV photons and two isocenters at the same sourceaxis distance.
Target dose coverage and homogeneity were given priority, whereby >95% of the PTV volume was covered by 95% of the prescribed dose and the maximum dose of the total plan (craniospinal plus boost) did not exceed 107%. Consideration was given to minimizing the OAR dose without compromising target coverage.
Statistical analyses
Dose distribution and Dose-Volume Histogram (DVH) data from 3D-CRT and VMAT included PTV dose coverage as V95% (the PTV receiving 95% of the prescribed dose), the Conformity Index CI (the ratio of V95% and total PTV), and the actual OAR dose. The plan homogeneity index HI was defined as the ratio of (D5%-D95%)/D50%. Analyses of the DVH for OAR were used to assess the radiation injury risk to specific organs; dose data were the mean and/or the maximum dose applied according to the relevance to each organ.
Results
Analysis of variables was carried out using the IBM SPSS statistics version 25.0. Characteristics were displayed descriptively. The distribution of HI, CI, D98%, D2%, D50%, dose in critical organs in each external irradiation technique were analyzed using statistical tests: Anova then Post Hoc Tests. We found interesting statistically significant results.
Planning target volume dosimetry
PTV coverage (D95%) was adequate for all plans. Figure 1 shows the 95% isodose for both VMAT and 3D-CRT in axial and coronal plans. Mean PTV dosimetry parameters between 3DCRT, and VMAT techniques in craniospinal irradiation can be seen in (Table 1). Conformity was superior with VMAT in all patients. The mean CI of 1, 08 for VMAT was lower than for 3D-RT (1, 34). Homogeneity Index was better in VMAT plan with a statistically significant difference (p=0, 04) while Dmax was lower with VMAT technique.
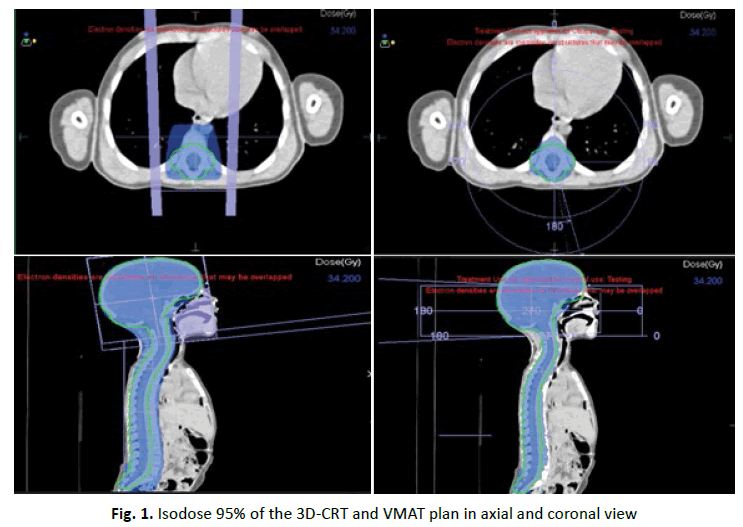
Figure 1: Isodose 95% of the 3D-CRT and VMAT plan in axial and coronal view
| Parameter | 3D-CRT | VMAT | P value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| D98% | 33.41 | 1.8 | 33.72 | 0.86 | 0.509 |
| D50% | 37.14 | 0.27 | 36.69 | 0.27 | 0.001 |
| D2% | 39.94 | 1.42 | 38 | 0.47 | 0.001 |
| Dmean | 37.16 | 0.26 | 36.51 | 0.32 | 0 |
| Dmax | 41.81 | 1.67 | 40.15 | 0.52 | 0.026 |
| HI | 0.12 | 0.05 | 0.07 | 0.01 | 0.043 |
| CI | 1.34 | 0.05 | 1.08 | 0.05 | 0 |
| PTV: Planning Target Volume, SD: Standard Deviation, HI: Homogeneity Index, CI: Conformity Index | |||||
Tab.1. Mean PTV dosimetry parameters among 3D-CRT, VMAT techniques in CSI
Organ at risk dosimetry
For dose to Organs At Risk (OAR), the 3D-CRT technique resulted in the highest maximum dose as expected which is seen at the Dose Volume Histogram DVH (Figure 2). VMAT plan provides reduced V20 and mean dose to almost all OAR delineated. The other interesting phenomenon that can be observed is that at low doses, the VMAT technique is delivering dose to larger volume of OAR. Table 2 summarizes some OAR doses and p-value of statically comparison of different dosimetric parameters of the dose-volume histogram.
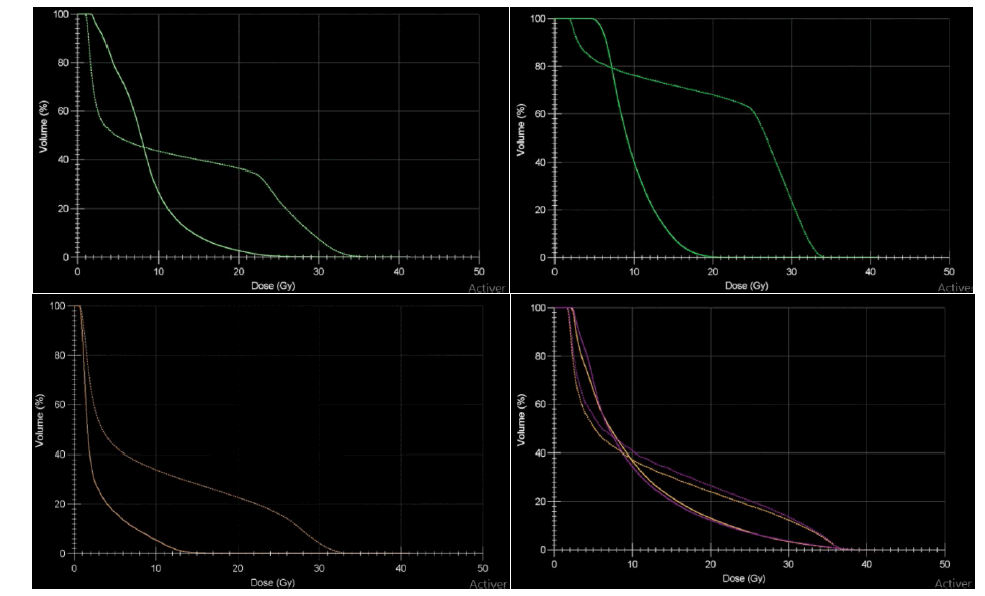
Figure 2: DVH comparison of VMAT and 3D-CRT. Light green: Heart, dark green: Liver, orange: Bladder, purple: kidneys right and left. 3D-CRT, VMAT
| OAR | Dmean(Gy) | Dmax (Gy) | V10 | V20 | V30 | V35 |
|---|---|---|---|---|---|---|
| Lungs | ||||||
| VMAT | 7.68 (2.19) | 37.38 (1.91) | 24.25 (11.19) | 8.64 (3.7) | 2.30 (1.9) | 0.41 (0.54) |
| 3D_CRT | 9.63 (1.95) | 37.85(1.75) | 30.69 (6.67) | 21.51 (5.5) | 10.48(3.69) | 2.29 (2.89) |
| P value | 0.019 | 0.807 | 0.021 | 0 | 0 | 0.13 |
| Right Kidney | ||||||
| VMAT | 5.71 (1.99) | 32.08(6.08) | 14.87 (8.95) | 4.26 (3.9) | 0.75 (1.28) | 0.15 (0.36) |
| 3D_CRT | 8.61 (3.01) | 35.37(2.11) | 27.68(10.66) | 16.53(8.35) | 7.07 (5.95) | 1.63 (3.9) |
| P value | 0.017 | 0.147 | 0.002 | 0.001 | 0.018 | 0.492 |
| Left Kidney | ||||||
| VMAT | 5.21 (2.04) | 30.13(5.29) | 13.15(10.54) | 3.16 (3.76) | 0.34 (0.74) | 0.04(0.12) |
| 3D_CRT | 7.36 (2.37) | 35.05(2.10) | 23.58 (9.50) | 66.71(168.51) | 4.74 (3.50) | 0.76(1.68) |
| P value | 0.034 | 0.007 | 0.013 | 0.305 | 0.003 | 0.361 |
| Heart | ||||||
| VMAT | 12.02(3.31) | 27.55(7.33) | 53.05(20.02) | 12.20 (15.71) | 1.47 (2.79) | 0.04 (0.10) |
| 3D_CRT | 20.19(3.14) | 33.31(2.03) | 74.33(10.44) | 65.54 (12.10) | 12.77(12.10) | 0.31(0.96) |
| P value | 0 | 0.03 | 0.001 | 0 | 0.088 | 0.59 |
| Oesophagus | ||||||
| VMAT | 27.10(3.99) | 34.47(2.84) | 98.79 (3.62) | 89.62 (10.40) | 34.29 (37.7) | 8.72(25.25) |
| 3D_CRT | 32.97(1.34) | 35.00(1.46) | 100.00(0.00) | 100.00 (0.00) | 96.91 (6.31) | 9.97(18.80) |
| P value | 0 | 0.843 | 0.381 | 0.001 | 0 | 0.984 |
| Thyroid | ||||||
| VMAT | 20.87(5.85) | 30.81(5.04) | 93.95(12.66) | 60.73 (39.00) | 7.28 (14.00) | 0.07 (0.14) |
| 3D_CRT | 30.84(1.59) | 34.46(1.11) | 100.00(0.00) | 99.86 (0.40) | 64.24(32.95) | 0.96 (2.67) |
| P value | 0.37 | 0.017 | 0.122 | 0.001 | 0.38 | 0.53 |
| Liver | ||||||
| VMAT | 7.35 (0.92) | 30.63(4.73) | 26.42 (7.24) | 3.71 (5.14) | 0.66 (1.41) | 0.03 (0.11) |
| 3D_CRT | 10.00(1.36) | 34.68(2.66) | 36.40 (4.17) | 29.40 (4.13) | 3.10 (3.58) | 0.16 (0.42) |
| P value | 0 | 0.046 | 0 | 0 | 0.114 | 0.599 |
Tab.2. Mean organ at risk dosimetry parameters among 3D-CRT and VMAT plans
Discussion
In children with medulloblastoma, which is a common childhood malignancy, long term survival was improved these last years because of therapeutic advances in radiation and chemotherapy [8]. The standard treatment for MBs includes surgical resection, followed by Radiotherapy (RT) to the craniospinal axis and then “boost” RT to the posterior fossa with or without chemotherapy.
That’s why it is very important to improve radiation therapy techniques to better cover target volumes and spare OARs to minimize long term toxicity, otherwise, late effects of radiation therapy such as somatic and carcinogenic effects may be observed during the followâ??up period [9].
VMAT radiation treatment techniques are gaining popularity due to their simplicity and faster treatment delivery time. VMAT- based CSI is increasingly being accepted as the choice of treatment technique over conventional techniques in clinics since it does not require any junction-shifts and it results in more conformal dose distribution [10, 11]. This planning study shows that VMAT may achieve a significant reduction in the non-target tissue integral dose delivered compared with 3D-CRT. VMAT additionally improves target dose conformity and normal tissue sparing compared with 3D-CRT. Compared with VMAT, there was poor conformity, small dose gradient and slow dose fall in 3D-CRT. As showed in Table 2, v10% of OAR was also high with VMAT, and dose dropped rapidly.
In the literature, several reports demonstrate improved CI and HI for the PTV and field-junctions by the use of modern radiotherapy techniques compared with 3D-CRT [12-14].
In a retrospective planning study comparing VMAT with conventional CSI in five patients, Lee, et al. [15] showed clinically relevant dose reductions to radiosensitive organs are achievable with VMAT. In particular, a reduction in the mean dose to the heart, esophagus, lenses, eyes, and optic nerves was observed, similar to the present study. Another planning study of VMAT [16], Intensity Modulated Radiation Therapy (IMRT), and 3D-CRT suggest that VMAT may be the optimal choice (compared with 3D-CRT) for treating the entire PTV based on sparing of the lenses, eyes, optic nerves, and cochlea that surround the cranial portion of the PTV as well as a reduction in integral dose with VMAT.
However, our study did not describe some other important points mainly the whole-body exposure to low doses with VMAT, the number of unit monitors which was correlated in some studies with the secondary induced cancers [17, 18]. This is related to the short course of follow up since the implementation of VMAT for CSI of MBs is recent in our centre.
Conclusion
In children patients requiring CSI, VMAT planning provides more homogenous target coverage while reducing the dose to multiple critical organs when compared with traditional 3D-CRT. This conformity comes with a trade-off of greater treatment times and low dose spread that introduces concern over the potential of secondary malignancies, especially for the VMAT technique.
The gain in target conformality with VMAT should be balanced with the spread of low doses to distant areas. This remains an open issue for the potential risk of secondary malignancies, and longer follow‑up is mandatory.
Acknowledgment
All the authors are thankful for being provided with the necessary facilities for the preparation of the manuscript.
Conflicts of Interest
The authors declare none.
References
- Packer RJ, Cogen P, Vezina G, Rorke LB. Medulloblastoma: clinical and biologic aspects. Neuro Oncol. 1999;1:232-250.
- Giordana MT, Schiffer P, Lanotte M, Girardi P, Chio A. Epidemiology of adult medulloblastoma. Int J Cancer. 1999;80:689-692.
- Frost PJ, Laperriere NJ, Wong CS, Milosevic MF, Simpson WJS, et al. Medulloblastoma in adults. Int J Radiat Oncol Biol Phys. 1995;32:951-957.
- Lynch CF, Hart MN, Jones MP. Medulloblastoma: a population-based study of 532 cases. J Neuropathol Exp Neurol. 1991;50:134-144.
- Merchant TE, Kun LE, Krasin MJ, Wallace D, Woo SY, et al. Multi-institution prospective trial of reduced-dose craniospinal irradiation (23.4 Gy) followed by conformal posterior fossa (36 Gy) and primary site irradiation (55.8 Gy) and dose-intensive chemotherapy for average-risk medulloblastoma. Int J Radiat Oncol Biol Phys. 2008;70:782-787.
- Laughton SJ, Merchant TE, Sklar CA, Kun LE, Fouladi M, et al. Endocrine outcomes for children with embryonal brain tumors after risk-adapted craniospinal and conformal primary-site irradiation and high-dose chemotherapy with stem-cell rescue on the SJMB-96 trial. J Clin Oncol.2008;25:1112-1118.
- Packer RJ, Zhou T, Holmes E, Vezina G, Gajjar A. Survival and secondary tumors in children with medulloblastoma receiving radiotherapy and adjuvant chemotherapy: results of Children’s Oncology Group trial A9961. Neuro Oncol. 2013;15:97-103.
- Helal A, Mostafa MF, Elsaka R, Fadel S. 3DCRT for posterior fossa; sparing of surrounding organs at risk. Alexendria J Med. 2014;50:311‑316.
- Paulino AC, Narayana A, Mohideen MN, Jeswani S. Posterior fossa boost in medulloblastoma: An analysis of dose to surrounding structures using 3‑dimensional (conformal) radiotherapy. Int J Radiat Oncol Biol Phys. 2000;46:281‑286.
- Strojnik A, Mendez I, Peterlin P. Reducing the dosimetric impact of positional errors in field junctions for craniospinal irradiation using VMAT. Rep Pract Oncol Radiother. 2016;21:232-239.
- Sarkar B, Munshi A, Manikandan A, Roy S, Ganesh T, et al. A low gradient junction technique of craniospinal irradiation using volumetric-modulated arc therapy and its advantages over the conventional therapy. Cancer/Radiothérapie. 2018;22:62-72.
- Parker W, Filion E, Roberge D, Freeman CR. Intensity-modulated radiotherapy for craniospinal irradiation: target volume considerations, dose constraints, and competing risks. Int J Radiat Oncol Biol Phys. 2007;69:251-257.
- Kusters JM, Louwe RJ, Kollenburg PG, Janssens GO, Lindert EJ, et al. Optimal normal tissue sparing in craniospinal axis irradiation using IMRT with daily intrafractionally modulated junction. Int J Radiat Oncol Biol Phys. 2011;81:1405-1414.
- St Clair WH, Adams JA, Bues M, Tarbell NJ, Kooy HM, et al. Advantage of protons compared to conventional X-ray or IMRT in the treatment of a ACTA ONCOLOGICA 9 pediatric patient with medulloblastoma. Int J Radiat Oncol Biol Phys. 2004;58:727-734.
- Lee YK, Brooks CJ, Bedford JL, Warrington AP, Saran FH. Development and evaluation of multiple isocentric volumetric modulated arc therapy technique for craniospinal axis radiotherapy planning. Int J Radiat Oncol Biol Phys. 2012;82:1006-1012.
- Studenski MT, Shen X, Yu Y, Xiao Y, Shi W, et al. Intensity-modulated radiation therapy and volumetric-modulated arc therapy for adult craniospinal irradiationda comparison with traditional techniques. Med Dosim. 2013;38:48-54.
- Followill D, Geis P, Boyer A. Estimates of whole‑body dose equivalent produced by beam intensity modulated conformal therapy. Int J Radiat Oncol Biol Phys. 1997;38:667‑672.
- Kry SF, Salehpour M, Followill DS, Stovall M, Kuban DA, et al. The calculated risk of fatal secondary malignancies from intensity‑modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2005;62:1195‑1203.



