Review Article - Onkologia i Radioterapia ( 2021) Volume 15, Issue 1
A review on permanent implants for prostate brachytherapy with comparison between stranded and loose seeds
Guangchao Wei1, Ping Jiang2, Chunxiao Li2, Shuhua Wei2, Yuliang Jiang2, Haitao Sun2 and Junjie Wang2*2Department of Radiation Oncology, Peking University 3rd Hospital, Beijing, China
Junjie Wang, Department of Radiation Oncology, Peking University 3rd Hospital, Beijing, China; ADDRESS:49 North Garden Rd., Haidian District Beijing, Postcode:100191, P.R. China, Email: junjiewang_edu@sina.cn
Received: 24-Nov-2020 Accepted: 15-Dec-2020 Published: 25-Dec-2020
Abstract
A systematic literature review to validate the conclusions regards to stranded seeds versus loose seeds. Published data for this review were identified by searching the PubMed databases. Based on these studies, in addition to the reduction of migration and displacement, stranded seeds may have dosimetric advantages, especially in dose homogeneity and coverage of peripheral target area due to its connection characteristics. Treatment margins could be the reason why there was no significant discrepancy between loose seeds and stranded seeds groups in some studies. But intraoperatively built custom links will prolong operation time, with the proficiency of technology, the prolonged time gradually decreases. However, the advantages of dosimetric could be dependent on the techniques used by the physicians. The outcomes of biochemical failure rate also support this hypothesis.
Keywords
Stranded seeds, loose seeds, IBCL, dosimetry, migration, bNED
Introduction
After Prostate-Specific Antigen (PSA) test was recommended for prostate cancer screening in the early 1990s, the clinical presentation of prostate cancer patients has shifted from a locally advanced disease to more organ-confined tumours [1]. Lowdose prostate brachytherapy is a standard treatment option for men with localized prostate adenocarcinoma. Both Permanent Seed Implantation (PI) and Permanent Seed Implantation combined with External Beam Radiation Therapy (PI+EBRT) have shown excellent tumour control [2-5]. In brachytherapy of prostate cancer, both Loose Seeds (LS) and stranded seeds have become standard techniques, as well as the preplanned approach and real-time intraoperative dosimetry system [6-8]. The use of stranded seeds for permanent seed implantation began in North America no later than 1998 [9]. At present, these two types of seeds are widely used in brachytherapy in Europe and North America. Stranded seeds have been used in brachytherapy in Japan since 2012 [10].
Both loose seeds and stranded seeds are used concurrently. At present, there are two types of Stranded Seeds (SS) commonly used in clinical practice. One type of SS has the seeds connected in a specific interval by the manufacturer. When used, it can be cut off at the connection to generate certain lengths or a given number of seeds. ISOCORD (EZAG, Berlin) is an example of stranded seeds. Another kind of stranded seeds is the so called Intraoperatively Built Custom Links (IBCL). The seed strands are built in the operation room, and the spacers between two seeds can be of different lengths. This literature review was carried out to validate the conclusions of stranded seeds versus loose seeds. Many studies have been carried out on prostate brachytherapy. The aim of this literature review was to identify the advantages and disadvantages of loose seeds and stranded seeds.
Common dosimetric parameters used for permanent implant evaluations are the D90, i.e., the minimal dose received by 90% of the prostate volume; V100/150/200, i.e., the volume receiving at least 100/150/200% of the prescribed dose; UD5/10/30/90, i.e., the dose received in 5%/10%/30%/90% of urethral volume; UV200, i.e., the urethral volume receiving at least 200% of the prescription dose; RD2 cc/0.1 cc/10, i.e., the dose in 2 cc/0.1 cc/10% of rectal volume; RV100/150, i.e., the rectal volume receiving at least 100/150% of the prescription dose; DHI, i.e., the dose homogeneity index, and COIN, i.e., the conformal index.
Materials and Methods
Published data used in this review were found by searching the PubMed databases (up to April 20, 2020). The terms “stranded seeds”, “loose seeds”, “IBCL”, and “prostate cancer” were used in the search. After the search, 44 studies were selected and read in details. Finally, 22 studies that met the inclusion criteria were selected, among which the study conducted by Ishiyama et al. [10] was a multi-institutional retrospective analysis. The inclusion criteria specified that only definitive studies on prostate cancer brachytherapy were selected.
Results
Dosimetric comparison of target volume
The dosimetric results for stranded seeds in prostate brachytherapy vs. loose seeds were different in different studies. A retrospective study of 13 hospitals and 426 patients in the IBCL group and 428 patients in the LS group conducted by Ishiyama et al. [10] showed that the dosimetric parameters in the IBCL group were not inferior to those in the LS group. There were significant differences in the V150 (LS:66.1% vs. IBCL:60.5%), D5(u) (LS:157.6% vs. IBCL:151.4%), and UV200 (LS:0.0043 ml vs. IBCL:0.0006 ml) at the planning phase. Post-implant dosimetry at one month also showed a significant difference in RV150 (LS:0.0452 ml vs. IBCL:0.0273 ml). In conclusion, this multiinstitutional retrospective study revealed no dosimetric demerits for using stranded seeds. A study conducted by Zauls et al. [11] enrolled 43 patients in the LS group (Pd-103:7, I-125:36) and 48 patients in the IBCL group (Pd-103:19, I-125:29). There was no significant difference in the D90 reported between the LS and IBCL groups, regardless of whether Pd-103 seeds or I-125 seeds were used.
Another study conducted by Ishiyama et al. [12] included 74 patients in the IBCL group and 66 patients in the LS group. Computed Tomography (CT) and plain radiography were acquired 1 day and 1 month after implantation, respectively, for evaluation. The primary endpoint in this study was the detection of a 5% difference in the dose to 90% of prostate volume on post-implant computed tomography 1 month after treatment. The dosimetric parameters including the primary endpoint did not differ significantly between the two groups.
The study conducted by Reed et al. [9] included 30 patients in the SS group and 32 patients in the LS group. The prescribed dose was 144 Gy. Magnetic Resonance Imaging (MRI), CT and plain radiography were performed on the day of implantation and 30 days later (Day 30). The results demonstrated that the dosimetric parameters were similar in both groups. However, the IBCL group showed a paradoxical trend toward lower V100 and D90 values (LS:96% vs. IBCL:94%, LS:178 Gy vs. IBCL:164 Gy), while the seeds in the LS group showed a significant local displacement compared to the seeds in the IBCL group. The authors attributed the lack of dosimetric effects partly to the small number of seeds lost in either group, and to their use of a high number of extra-prostatic seeds and high treatment margins.
The study conducted by Inada et al. [13] included 37 patients in both LS and IBCL groups. Remarkably, the decrease of D90 from next day to 30-day dosimetric evaluation in the IBCL group was significantly smaller than that in the LS group (IBCL:- 1.16% vs. LS:-4.17%). Thus, IBCL helped achieve high CTV coverage and prostate homogeneity in intraoperative planning, and prevented the decrease in prostate D90 in 1-month postimplant dosimetry.
In the study by Hirose et al. [14], low-and intermediate-risk patients received permanent I-125 seed PI alone with the prescribed dose of 160 Gy. There were 14 patients in the LS group and 10 patients in the IBCL group. High-risk patients received PI at 110 Gy, followed by External Beam Radiation Therapy (EBRT) at 45 Gy (PI+ EBRT), with 25 patients in the LS group and 13 patients in the IBCL group. Dosimetric evaluation was conducted on the next day and 30 days after implantation. The dose homogeneity index (DHI=(v100-v150)/v100) also failed to show a significant difference. For the patients who received PI +EBRT therapy, the IBCL group was significantly superior in terms of D90, V150, V200, V250, HI, UD5 and UD30. Therefore, it was only confirmed that IBCL had dosimetric advantages over LS at a lower prescribed dose of 110 Gy. The study conducted by Katayama et al. [15] included 32 patients in both LS and IBCL groups. MRI and CT were performed on 30 days after implantation. The IBCL group showed a significant superiority in V100 (IBCL:95.3% vs. LS:89.7%) and D90 (IBCL:169.7 Gy vs. LS:152.6 Gy) compared to the LS group.
Kaneda et al. [16] conducted a study on the plan reproducibility of IBCL compared to LS. The primary endpoint was the mean of the absolute change in the minimum dose received by 90% of the prostate volume. The IBCL group included 39 patients and the LS group included 37 patients, and both groups were given a prescribed dose of 110 Gy. The D90 of the LS group changed more than that in the IBCL group (LS:6.95% vs. IBCL: -0.41%). The IBCL group showed decreased post-operative D90 (IBCL:118.8% vs. LS:127.2%), V150 (IBCL:51.7% vs. LS:66.7%), and RV100 (IBCL:0.44 ml vs. LS:0.61 ml) compared to the LS group. The study conducted by Major et al. [17] included 79 patients in the LS group and 126 patients in the SS group. Dosimetric evaluation was conducted immediately after implantation. Based on V100 and D90, the SS group showed a significant superiority compared to the LS group (SS:98% vs. LS:96%, SS:172 Gy vs. LS:166 Gy), but more conformal dose distributions were observed with LS (LS: COIN=0.70 vs. SS: COIN=0.63). 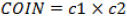 , c1=PTVref / VPTV, C2=PTVref / Vref. The PTVref is the volume of the PTV receiving a dose equal to or greater than the reference dose (prescribed dose). The V_PTV is the volume of the PTV and V_ref is the volume receiving a dose equal to or greater than the reference dose. The dose homogeneity did not differ significantly between the two groups, and the LS group had a smaller dose in the urethra and rectum. The reason was the difference in the flexibility of loading patterns between the two techniques.
, c1=PTVref / VPTV, C2=PTVref / Vref. The PTVref is the volume of the PTV receiving a dose equal to or greater than the reference dose (prescribed dose). The V_PTV is the volume of the PTV and V_ref is the volume receiving a dose equal to or greater than the reference dose. The dose homogeneity did not differ significantly between the two groups, and the LS group had a smaller dose in the urethra and rectum. The reason was the difference in the flexibility of loading patterns between the two techniques.
The study conducted by Herbert et al. [18] included 227 patients in the LS group and, 173 patients in the SS group, and both groups were given a prescribe dose of 144 Gy. The SS group showed advantages in terms of D90 and V100: D90 was 106.5% (percentage of prescribed dose) in the SS group and 102.5% in the LS group, while V100 was 93.0% in the SS group and 91.3% in the LS group.
The study conducted by Saibish kumar et al. [19] included 20 patients in both LS and IBCL groups, and both groups were given a prescribed dose of 145 Gy. MRI and CT were performed right after implantation and 7/30 days after implantation. After implantation, the LS group had larger V100 (LS:93.2% vs. SS:89.2%) and D90 (LS:153.9 Gy vs. SS:144.2 Gy), but this discrepancy was not significant 7/30 days after implantation. The study conducted by Kudchadker et al. [20] included 100 patients in the LS group and 81 patients in the SS group, and both groups were given a prescribe dose of 145 Gy. The total implanted activity and the number of seeds used were significantly lower in the SS group than those in the LS group. The reduction in activity in the SS group was approximately 23% for a 20 cm3 prostate and approximately 15% for a 60 cm3 prostate. When the activity between the two groups was equivalent, the SS treatment covered a larger treatment volume with the prescribed dose. The authors attributed this to the better position distribution and dose homogeneity of SS. Due to local displacement of LS, more seeds were needed to cover the periphery of PTV. The study conducted by Lee et al. [21] included 20 patients in both groups given a prescribe dose of 144 Gy. CT was performed 30 days after implantation. The SS group showed advantages in V100 (SS:94.1% vs. LS:86.5%) and D90 (SS:164.2 Gy vs. LS:132.1 Gy). All patients in the SS group had D90 above 140 Gy, but only 7 patients in the LS group had D90 above 140 Gy. The study conducted by Kaplan et al. [22] included 4 patients in both groups. The LS group showed the advantage in D90. The author believed that SS had no dosimetric benefits, but the sample size in this study was small. The study conducted by Laimonas et al. [23] included 106 patients in the LS group and 124 patients in the SS group, and both groups were given a prescribed dose of 160 Gy. CT performed 30 days after implantation was used for dosimetric evaluation. SS had lower D90 (SS:177.9 Gy vs. LS:184.7 Gy). No significant discrepancy was seen in V100. SS also had better dose homogeneity. The findings of the literature search are summarized in Table 1.
Tab.1. Target volume dosimetric parameters
| Study(ref) | n | Phase | Parament | SS | IBCL | LS | P | Advantage |
|---|---|---|---|---|---|---|---|---|
| Ishiyama [10] | 854 | Planing | PD90(%) | 123.7 ± 11.9 | 124.3 ± 13.6 | NS | ||
| PV100(%) | 97.1 ± 4.4 | 96.5 ± 3.8 | NS | |||||
| PV150(%) | 60.5 ± 14.8 | 66.1 ± 14.0 | <0.001 | + | ||||
| Day 30 | PD90(%) | 118.1 ± 14.8 | 119.3 ± 16.5 | NS | ||||
| PV100(%) | 95.5 ± 5.2 | 95.5 ± 4.5 | NS | |||||
| PV150(%) | 60.2 ± 15.5 | 67.6 ± 13.4 | <0.001 | + | ||||
| Zauls [11] | 91 | Day 0 | PD90(%)(Pd103) | 114.5 ± 7.5 | 113.1 ± 10.5 | NS | ||
| PD90(%)(I125) | 173.4 ± 8.9 | 170.0 ± 10.7 | NS | |||||
| Day 30 | PD90(%)(Pd103) | 104.0 ± 10.9 | 98.2 ± 8.8 | NS | ||||
| PD90(%)(I125) | 165.1 ± 11.6 | 164.5 ± 12.8 | NS | |||||
| Ishiyama [12] | 140 | Day 1 | PD90(Gy) | 153.1 ± 14.7 | 151.7 ± 14.2 | NS | ||
| PV100(%) | 92.0 ± .6.3 | 91.2 ± 6.0 | NS | |||||
| PV150(%) | 46.1 ± 10.4 | 46.3 ± 11.4 | NS | |||||
| Day 30 | PD90(Gy) | 174.4 ± 19.9 | 170.7 ± 18.5 | NS | ||||
| PV100(%) | 96.6 ± 3.6 | 95.7 ± 3.7 | NS | |||||
| PV150(%) | 60.4 ± 12.6 | 62.1 ± 12.9 | NS | |||||
| Reed [9] | 64 | Day 0 | PV100(%) | 95 | 95 | 0.96 | ||
| PD90(Gy) | 169 | 169 | 0.88 | |||||
| Day 30 | PV100(%) | 94 | 96 | 0.012 | - | |||
| PD90(Gy) | 164 | 178 | 0.058 | |||||
| Inada [13] | 74 | Day 0 | PV100(%) | 97.7 ± 2.10 | 96.9 ± 1.88 | 0.13 | ||
| PV150(%) | 54.8 ± 8.08 | 59.6 ± 9.76 | 0.027 | + | ||||
| PD90(%) | 120.9 ± 9.93 | 119.6 ± 8.20 | 0.55 | |||||
| CTVV100(%) | 88.1 ± 4.17 | 85.6 ± 4.72 | 0.019 | + | ||||
| CTVV150(%) | 43.9 ± 6.63 | 45.5 ± 7.88 | 0.34 | |||||
| CTVD90(%) | 98.5 ± 8.24 | 92.6 ± 8.00 | 0.0033 | + | ||||
| Day 30 | PV100(%) | 96.9 ± 2.87 | 95.2 ± 2.92 | 0.02 | + | |||
| PV150(%) | 57.1 ± 11.27 | 64.5 ± 10.55 | 0.0051 | - | ||||
| PD90(%) | 119.8 ± 11.65 | 115.5 ± 10.14 | 0.1 | |||||
| CTVV100(%) | 85.6 ± 4.34 | 81.7 ± 5.27 | 0.0012 | + | ||||
| CTVV150(%) | 31.5 ± 8.14 | 35.7 ± 7.68 | 0.046 | + | ||||
| CTVD90(%) | 94.2 ± 9.34 | 86.5 ± 8.52 | <0.001 | + | ||||
| Hirose [14] | 62 | Day 0 | PD90(Gy)(PI) | 209.1 ± 12.6 | 195.8 ± 10.3 | 0.009 | + | |
| CTVHI(PI) | 33.9 ± 10.0 | 31.1 ± 10.0 | 0.509 | |||||
| PD90(Gy)(PI+EBRT) | 149.1 ± 9.1 | 153.0 ± 11.8 | 0.297 | |||||
| CTVHI(PI+EBRT) | 25.4 ± 12.5 | 18.9 ± 8.8 | 0.067 | |||||
| PV200(%)(PI+RBRT) | 33.4 ± 8.6 | 40.8 ± 9.0 | 0.013 | + | ||||
| PV250(%)(PI+RBRT) | 5.7 ± 5.2 | 12.0 ± 7.2 | 0.01 | + | ||||
| Day 30 | PD90(Gy)(PI) | 190.1 ± 15.2 | 190.3 ± 23.6 | 0.9783 | ||||
| CTVHI(PI) | 41.3 ± 10.9 | 33.6 ± 15.5 | 0.191 | |||||
| PV150(%)(PI) | 68.3 ± 10.3 | 57.3 ± 11.1 | 0.006 | - | ||||
| PV200(%)(PI) | 25.7 ± 6.1 | 29.7 ± 5.7 | 0.030 | + | ||||
| PD90(Gy)(PI+EBRT) | 134.5 ± 12.1 | 148.3 ± 12.7 | 0.004 | - | ||||
| CTVHI(PI+EBRT) | 35.7 ± 11.7 | 18.7 ± 9.3 | <0.001 | + | ||||
| PV150(%)(PI+RBRT) | 62.8 ± 12.9 | 80.2 ± 10.0 | 0.001 | + | ||||
| PV200(%)(PI+RBRT) | 28.0 ± 6.5 | 47.5 ± 12.7 | 0.001 | + | ||||
| PV250(%)(PI+RBRT) | 8.3 ± 5.4 | 17.4 ± 12.9 | 0.020 | + | ||||
| Katayama [15] | 64 | Planing | PD90(Gy) | 175.6 ± 6.7 | 179.6 ± 7.3 | 0.024 | - | |
| PV100(%) | 99.4 ± 0.6 | 99.5 ± 0.7 | 0.96 | |||||
| PV150(%) | 56.3 ± 6.3 | 59.6 ± 7.1 | 0.051 | |||||
| Day 30 | PD90(Gy) | 180.7 ± 12.7 | 178.1 ± 15.4 | 0.29 | ||||
| PV100(%) | 98.2 ± 1.4 | 97.0 ± 2.4 | 0.057 | |||||
| PV150(%) | 69.2 ± 9.9 | 68.8 ± 11.3 | 0.88 | |||||
| Kaneda [16] | 76 | Day 0 | PD90(%) | 119.2 ± 5.5 | 120.3 ± 5.1 | 0.40 | ||
| PV100(%) | 98.8 ± 1.1 | 98.4 ± 1.4 | 0.14 | |||||
| PV150(%) | 43.0 ± 7.5 | 47.7 ± 6.4 | <0.01 | + | ||||
| Day 30 | PD90(%) | 118.8 ± 9.2 | 127.2 ± 11.4 | <0.01 | - | |||
| PV100(%) | 98.0 ± 1.9 | 98.7 ± 1.4 | 0.06 | |||||
| PV150(%) | 51.7 ± 11.4 | 66.7 ± 13.2 | <0.01 | + | ||||
| Major [17] | 205 | Day 28 | PV100(%) | 98 ± 2 | 96 ± 2 | <0.05 | + | |
| PV90(%) | 99 ± 1 | 98 ± 6 | <0.05 | + | ||||
| PV150(%) | 60 ± 6 | 58 ± 7 | 0.05 | - | ||||
| PV200(%) | 25 ± 6 | 30 ± 6 | <0.05 | + | ||||
| PD90(%) | 119 ± 5 | 115 ± 5 | <0.05 | + | ||||
| PD100(%) | 76 ± 10 | 69 ± 11 | <0.05 | + | ||||
| DHI | 0.38 ± 0.06 | 0.39 ± 0.07 | 0.21 | |||||
| COIN | 0.63 ± 0.04 | 0.70 ± 0.05 | <0.05 | - | ||||
| Herbert [18] | 1500 | Day 28 | PD90(%) | 106.7 ± 12.1 | 102.2 ± 13.8 | <0.0001 | + | |
| PV100(%) | 92.2 ± 4.9 | 89.2 ± 8.0 | <0.0001 | + | ||||
| PV150(%) | 57.0 ± 11.2 | 51.3 ± 14.1 | <0.0001 | - | ||||
| PV200(%) | 25.2 ± 8.9 | 23.0 ± 9.2 | 0.0004 | - | ||||
| Saibishkumar [19] | 20 | Day 0 | PV100(%) | 89.2 ± 4.5 | 93.2 ± 3.8 | 0.004 | - | |
| PD90(Gy) | 144.2 ± 8.0 | 153.9 ± 11.0 | 0.003 | - | ||||
| PV150(%) | 38.6 ± 6.6 | 44.8 ± 7.8 | 0.01 | + | ||||
| Day 7 | PV100(%) | 92.8 ± 2.7 | 93.1 ± 4.6 | 0.8 | ||||
| PD90(Gy) | 152.5 ± 9.0 | 155.7 ± 13.5 | 0.4 | |||||
| PV150(%) | 46.0 ± 7.0 | 49.2 ± 8.6 | 0.2 | |||||
| Day 30 | PV100(%) | 95.6 ± 3.2 | 94.9 ± 4.0 | 0.5 | ||||
| PD90(Gy) | 166.9 ± 14.0 | 163.7 ± 16.9 | 0.3 | |||||
| PV150(%) | 58.3 ± 9.7 | 60.4 ± 10.4 | 0.5 | |||||
| Kudchadker [20] | activity/volume | 1.15 ± 0.14 | 1.42 ± 0.20 | <0.0001 | + | |||
| Lee [21] | 40 | Day 30 | PV100(%) | 94.10 ± 2.9 | 86.54 ± 3.7 | <0.001 | + | |
| PV90(%) | 96.63 ± 2.2 | 90.43 ± 3.2 | <0.001 | + | ||||
| PV80(%) | 98.50 ± 1.3 | 94.12 ± 2.6 | <0.001 | + | ||||
| PD90(Gy) | 164.2 ± 17.3 | 132.13 ± 11.6 | <0.001 | + | ||||
| No.D90>140 Gy | 20 | 7 | <0.001 | + | ||||
| Kaplan [22] | 8 | Day 30 | PD90(%) | 92.7 | 110.0 | NS | ||
| PV100(%) | 89.6 | 89.8 | NS | |||||
| Laimonas [23] | 186 | Day 0 | PV100(%) | 96.8 ± 1.3 | 96.1 ± 1.7 | <0.001 | + | |
| PV150(%) | 54.8 ± 5.4 | 61.4 ± 5.6 | <0.001 | + | ||||
| PV200(%) | 22.6 ± 3.9 | 27.3 ± 5.4 | <0.001 | + | ||||
| PD90(Gy) | 185.7 ± 7.5 | 186.4 ± 9.6 | 0.595 | |||||
| PD50(Gy) | 247.0 ± 24.5 | 262.0 ± 12.8 | <0.001 | + | ||||
| DHI | 43.4 ± 5.6 | 36.1 ± 5.3 | <0.001 | + | ||||
| Day 30 | PV100(%) | 94.9 ± 3.2 | 95.5 ± 2.4 | 0.206 | ||||
| PV150(%) | 53.2 ± 10.0 | 65.3 ± 8.1 | <0.001 | + | ||||
| PV200(%) | 24.3 ± 6.9 | 35.1 ± 7.5 | <0.001 | + | ||||
| PD90(Gy) | 177.9 ± 12.7 | 184.7 ± 15.0 | 0.002 | - | ||||
| PD50(Gy) | 247.4 ± 20.7 | 274.2 ± 25.3 | <0.001 | - | ||||
| DHI | 44.0 ± 9.8 | 31.8 ± 7.3 | <0.001 | + |
Values are given as mean ± SD,
if SD is available No.D90>140 Gy: Number of patient with D90>140 Gy
P: prostate volume,
CTV: clinical target volume, n: number of cases
Tissue oedema
Saibishkumar et al.[24] Found that the tissue oedema caused by the SS was less than that caused by the LS. At all-time points (Day 0, 7, and 30), the mean oedema factor (percentage increase in prostate volume compared with pre-implant ultrasound volume) was higher for LS than for SS, but no statistical significance was reached with this small sample size: Day 0 (33.9% vs. 31.8%), Day 7 (23.3% vs. 19.3%), and Day 30 (7.2% vs. 2.2%). The study of Ishiyama et al. [10] implied that the IBCL may reduce prostate oedema (Pre-operation prostate volume LS:25.7 mm, IBCL:25.9 mm; post-operation prostate volume LS:27.5 mm, IBCL:26.9 mm). Pinkawa et al. [25] explored the relationship between dose and tissue oedema. 51 patients received SS implant with a prescribed dose of 145 Gy. CT scans were performed before implantation, 1 day after implantation and 30 days after implantation. The mean volume of prostate was 38 cc, 50 cc and 40 cc, respectively. The D90 was 138 Gy and154 Gy, the V100 was 87% and 91%, the V150 was 54% and 65%, the rectum V100 was 0.4 cc and 1.1 cc, the rectum V50 was 4.2 cc and 6.2 cc, the rectum D2 cc was 90 Gy and 113 Gy, and the rectum D0.1 cc was 169 Gy and 220 Gy, respectively, in dosimetric evaluations carried out 1 day and 30 days after implantation. In summary, dosimetric parameters increased when postoperative tissue oedema was relieved. The study also found that the displacement of SS was strongly related to the movement of PTV contour relative to the pelvis (PTV was set according to prostate contour in real time). The studies above suggested that stranded seeds could involve less oedema and organ movement, thus reducing their influence on dose. However, these results were not consistent with those in another study conducted by Laughlin et al.[26], which included 28 patients in the stranded seeds group, which did not support the hypothesis that the change of dosimetric parameter is related to the relief of tissue oedema. In this study, the patients underwent MRI and CT scans on day 0 and day 14 after implantation. The D90 in 27 of 28 patients changed between Day 0 and 14. No relationship was found between the change in prostate volume and the change on D90 (R2=0.01). A paradoxic dosimetric result was noted in 11 of 28 patients. The rectal dose increased in 23 of 28 patients, with a >30 Gy change in 6 patients. The external sphincter D90 increased in 19 of 28 patients, with a >50 Gy increase in 6 patients. Furthermore, the movement of prostate position on the vertical axis was inconsistent with the movement of isodose line on the vertical axis.
Biochemical failure rate
Some studies compared the biochemical failure rates between the groups of stranded seeds and loose seeds. According to the study conducted by Herbert et al. [18] and based on the Phoenix definition, the rate of biochemical no evidence of disease (bNED) was estimated to be 93.5% at 7 years for patients treated with LS and 94.0% for patients treated with SS. Using the PSA<0.4 ng/mL definition, the estimated rates were 91.3% and 91.9% for LS and SS, respectively. The cutoff date was selected to allow a minimum of 4-year follow-up. The median follow-up time was 66 months (IQR 45-72 months). 1,500 patients were included in this study (LS:227, SS:1173).
The study conducted by Hinnen et al. [27] included 358 patients in the LS group and 538 patients in the SS group, which were given a prescribed dose of 144 Gy. The median follow-up time was 42 months (IQR 24-60 months). In the SS group, D90 was 184 Gy and 153 Gy, respectively, on day 0 and day 30 after implantation. In the LS group, D90 was 183 Gy and 170 Gy, respectively, on day 0 and day 30 after implantation. Based on the Phoenix definition, the rate of bNED was 86.1% for patients treated with SS and 89.5% for patients treated with LS after 5 years. When adjusted for possible confounding factors in a Cox-regression analysis, the seed type was significantly associated with biochemical failure, with a 43% risk reduction for loose seeds versus stranded seeds.
Seed migration
A consensus was reached among different researchers regarding seed migration and local displacement. Almost all researchers agreed that SS or IBCL would reduce seed migration compared to LS [9,10,12,13,14,15,16,28,29]. Vassiliev et al. [30] investigated local displacement of SS around the urethra in a study involving 10 patients. The movement of SS particles in all directions was less than 1 mm on the dosimetry done 30 days after operation.
The study of Saibish kumar et al. [19] Showed 13 events of seed migration in 5 patients (0.6%) of the LS group. Five seeds migrated to the lungs in 4 patients and one seed was lost through the urinary tract. No seed migrated to the lungs in the SS group, but 24 seeds migrated in 5 patients (1.1%) and 22 seeds was lost through the urinary tract in 5 patients. The finding of increased seed loss through the urinary tract in the SS cohort was unexpected. The authors attributed this phenomenon to some technical issues. The study of Al-Qaisieh et al. [31] included 238 patients receiving the SS implant. In this study, 100 patients underwent X-ray examinations on day 55 after implantation on average, and no seed migration to the lung was found. According to the study of Lee et al. [21], seed migration was observed in 2 patients (10%) treated with LS. Seed migration was not seen in any patients treated with SS.
A different result was seen in the study of Kaplan et al. [22], with an average seed movement of 3.1 mm in the LS group and 3.7 mm in the SS group, but their sample size was small. The findings of the literature search are summarized in Table 2.
Tab.2. Seed migration
| Study(ref) | n | Phase | Parament | SS | IBCL | LS | P | Advantage |
|---|---|---|---|---|---|---|---|---|
| Ishiyama [10] | 854 | Day 30 | Lungs (P) | 1 (0.2%) | 39 (9.1%) | <0.001 | + | |
| Lungs (S/P) | 1 | 1.51 | ||||||
| Abdomino-pelvis (P) | 8 (1.9%) | 77 (18.0%) | <0.001 | + | ||||
| Abdomino-pelvis (S/P) | 1 | 1.68 | ||||||
| Total (P) | 9 (2.1%) | 98 (22.9%) | <0.001 | + | ||||
| Total (S/P) | 1 | 1.92 | ||||||
| Ishiyama [12] | 140 | Day 1 | Total (P) | 0 (0%) | 29 (43.9%) | <0.001 | + | |
| Chest (P) | 0 (0%) | 5 (7.6%) | ||||||
| Abdomino-pelvis (P) | 0 (0%) | 26 (39.4%) | ||||||
| Seminal vesicle (P) | 0 (0%) | 8 (12.1%) | ||||||
| Day 30 | Total (P) | 0 (0%) | 36 (54.5%) | <0.001 | + | |||
| Chest (P) | 0 (0%) | 20 (30.3%) | ||||||
| Abdomino-pelvis (P) | 0 (0%) | 26 (39.4%) | ||||||
| Seminal vesicle (P) | 0 (0%) | 10 (15.2%) | ||||||
| Reed [9] | 64 | Day 30 | Total (P) | 7 (23%) | 15 (47%) | <0.053 | + | |
| Total (S/P) | 0.4 | 1.1 | 0.062 | + | ||||
| Inada [13] | 74 | Day 30 | Total (P) | 2 (5%) | 15 (41%) | <0.001 | + | |
| Chest (P) | 0 (0%) | 2 (5%) | ||||||
| pelvis (P) | 0 (0%) | 8 (22%) | ||||||
| Seminal vesicle (P) | 2 (5%) | 6 (16%) | ||||||
| Hirose [14] | 62 | Day 30 | Total (P) (PI) | 0 (0%) | 4 (28.6%) | |||
| Total (P) (PI+EBRT) | 0 (0%) | 6 (24.0%) | ||||||
| Katayama [15] | 64 | Day 30 | Total (P) | 2 (6.3%) | 21 (65.6%) | <0.001 | + | |
| Chest (P) | 1 (3.1%) | 15 (46.9%) | ||||||
| Abdomino-pelvis (P) | 0 (0%) | 15 (46.9%) | ||||||
| Seminal vesicle (P) | 0 (0%) | 6 (18.8%) | ||||||
| Distal (P) | 1 (3.1%) | 1 (3.1%) | ||||||
| Kaneda [16] | 76 | Day 30 | Lungs (P) | 0 (0%) | 3 (8%) | 0.07 | + | |
| Merrell [28] | 957* | Day 124** | Lungs (P) | 5 (0.9%) | 178 (45.5%) | <0.0001 | + | |
| Lungs (S/P) | 1 | 1.9 | ||||||
| Birckhead [29] | 137 | Day 109** | Total (P) | 38 (28%) | ||||
| Total (S/P) | 2.7 | |||||||
| Saibishkumar [19] | 40 | Day 30 | Bladder (P) | 1 (5%) | 1 (5%) | |||
| Bladder (S/P) | 1 | 1 | ||||||
| Urine (P) | 5 (25%) | 1 (5%) | ||||||
| Urine (S/P) | 4.4 | 1 | ||||||
| Pelvis (P) | 1 (5%) | 0 (0%) | ||||||
| Pelvis (S/P) | 1 | 0 | ||||||
| Lungs (P) | 0 (0%) | 4 (20%) | ||||||
| Lungs (S/P) | 0 | 1.25 | ||||||
| Ejaculate (P) | 0 (0%) | 1 (5%ï¼? | ||||||
| Ejaculate (S/P) | 0 | 6 | ||||||
| Total (P) | 6 (30%) | 5 (25%) | ||||||
| Total (S/P) | 4 | 2.6 | ||||||
| Qaisieh [31] | 100 | Day 53** | Lungs (P) | 0 (0%) | ||||
| Lungs (S/P) | 0 | |||||||
| Lee [21] | 40 | Day 30 | Total (P) | 0 (0%) | 2 (10%) |
P: Patients with seed migration,
S/P: Seeds per patient with seed migration,
*: exclude mixed implant group,
**: median number of days, Some studies did not provide P value, and some studies did not include LS group,
n: number of cases
Organs at risk
A study of Ishiyama et al. [10] showed that the RV150 was significantly lower in the IBCL group compared with the LS group (IBCL: 0.0273 ml vs. LS:0.0452 ml) 1 month after implantation. Another study of Ishiyama et al. [12] showed no significant difference in acute toxicity 9 months after implantation, and no significant difference in dosimetry between postoperative dosimetry done on day 0 and day 30.
Reed et al. [9] did not find significant difference in dosimetry one month after implantation.
Inada et al. [13] found that one month after implantation, the reduction of RD2 cc was more significant in the IBCL group. The authors also believed that the lower V150 in the IBCL group might help reduce the complications of the urinary and gastrointestinal systems, although this study lacked long-term follow-up data. According to the study of Hirose et al.[14], the patients in the IBCL group who received PI treatment had lower RV100 at 30 days after implantation, and the patients who received PI+EVRT treatment had lower UD5 and UD30.
Katayama et al. [15] found that the IBCL group showed a paradoxical trend toward lower UD90 values one month after implantation, and this trend persisted during the planning phase as well. Kaneda et al.[16] found that the RV100 in the linked seed group was significantly lower than that in the loose seed group. According to the study of Major et al. [17], the all dosimetric parameters related to the dose to urethra and rectum were significantly lower in the LS group. In the study of Laimonas et al. [23], UD30, UD10 and RV100 were lower in the SS group compared to the LS group on both day 0 and day 30 after implantation. The findings of the literature search are summarized in Table 3.
Tab.3. Organs at risk
| Study(ref) | n | Phase | Parament | SS | IBCL | LS | P | Advantage |
|---|---|---|---|---|---|---|---|---|
| Ishiyama [10] | 854 | Planing | UD5(%) | 151.4 ± 27.2 | 157.6 ± 31.7 | 0.05 | + | |
| UD90(%) | 102.4 ± 24.2 | 99.1 ± 29.3 | NS | |||||
| UV200(ml) | 0.0006 ± 0.0029 | 0.0043 ± 0.0205 | <0.01 | + | ||||
| RV100(ml) | 0.1567 ± 0.2728 | 0.1467 ± 0.2432 | NS | |||||
| RV150(ml) | 0.0086 ± 0.0392 | 0.0050 ± 0.0150 | NS | |||||
| Day 30 | UD5(%) | 167.2 ± 38.5 | 172.4 ± 38.3 | NS | ||||
| UD90(%) | 108.2 ± 24.3 | 105.0 ± 26.8 | NS | |||||
| UV200(ml) | 0.0050 ± 0.0173 | 0.0043 ± 0.0147 | NS | |||||
| RV100(ml) | 0.0050 ± 0.0173 | 0.4245 ± 0.0147 | NS | |||||
| RV150(ml) | 0.0273 ± 0.0576 | 0.0452 ± 0.1001 | <0.05 | + | ||||
| Ishiyama [12] | 140 | Day 1 | RV100(ml) | 0.15 ± 0.26 | 0.15 ± 0.27 | NS | ||
| RV150(ml) | 0.012 ± 0.04 | 0.014 ± 0.04 | NS | |||||
| UV200(ml) | 0.004 ± 0.01 | 0.005 ± 0.02 | NS | |||||
| UD90(Gy) | 132.2 ± 22.4 | 130.8 ± 17.3 | NS | |||||
| UD30(Gy) | 173.3 ± 16.7 | 172.1 ± 16.9 | NS | |||||
| UD5(Gy) | 199.6 ± 29.8 | 197.7 ± 32.5 | NS | |||||
| Day 30 | RV100(ml) | 0.47 ± 0.55 | 0.51 ± 0.64 | NS | ||||
| RV150(ml) | 0.03 ± 0.04 | 0.05 ± 0.11 | NS | |||||
| UV200(ml) | 0.007 ± 0.018 | 0.006 ± 0.016 | NS | |||||
| UD90(Gy) | 160.1 ± 22.7 | 158.3 ± 23.3 | NS | |||||
| UD30(Gy) | 203.2 ± 22.9 | 206.8 ± 26.1 | NS | |||||
| UD5(Gy) | 236.8 ± 40.5 | 239.4 ± 47.0 | NS | |||||
| Reed [9] | 64 | Day 0 | RV100(ml) | 0.81 | 0.56 | 0.25 | ||
| Day 30 | RV100(ml) | 1.91 | 1.96 | 0.8 | ||||
| Inada [13] | 74 | Day 0 | UD10(%) | 131.1 ± 9.15 | 129.7 ± 10.32 | 0.56 | ||
| RD2 cc(%) |
66.8 ± 8.70 | 64.5 ± 5.39 | 0.17 | |||||
| Day 30 | UD10(%) | 141.2 ± 14.10 | 145.5 ± 15.95 | 0.23 | ||||
| RD2 cc(%) | 61.0 ± 10.18 | 64.1 ± 11.15 | 0.23 | |||||
| Hirose [14] | 62 | Day 0 | UD30(Gy)(PI) | 223.7 ± 14.3 | 226.9 ± 23.4 | 0.398 | ||
| UD5(Gy)(PI) | 235.1 ± 115.1 | 245.7 ± 37.3 | 0.236 | |||||
| RV100(ml)(PI) | 0.30 ± 0.32 | 0.07 ± 0.16 | 0.026 | + | ||||
| UD30(Gy)(PI+EBRT) | 166.0 ± 14.2 | 174.5 ± 14.3 | 0.038 | + | ||||
| UD5(Gy)(PI+EBRT) | 184.4 ± 20.4 | 190.9 ± 19.4 | 0.29 | |||||
| RV100(ml)(PI+EBRT) | 0.13 ± 0.36 | 0.42 ± 0.44 | 0.074 | |||||
| Day 30 | UD30(Gy)(PI) | 219.9 ± 23.6 | 248.9 ± 35.8 | 0.159 | ||||
| UD5(Gy)(PI) | 255.6 ± 46.7 | 278.6 ± 44.0 | 0.488 | |||||
| RV100(ml)(PI) | 0.23 ± 0.35 | 0.14 ± 0.13 | 0.119 | |||||
| UD30(Gy)(PI+EBRT) | 165.2 ± 23.2 | 195.1 ± 32.7 | 0.003 | + | ||||
| UD5(Gy)(PI+EBRT) | 200.3 ± 32.6 | 230.1 ± 36.3 | 0.008 | + | ||||
| RV100(ml)(PI+EBRT) | 0.31 ± 0.20 | 0.29 ± 0.35 | 0.306 | |||||
| Katayama [15] | 64 | Planing | RV100(ml) | 0.40 ± 0.24 | 0.34 ± 0.22 | 0.38 | ||
| RV150(ml) | 0.01 ± 0.02 | 0.01 ± 0.001 | 0.29 | |||||
| UD90(Gy) | 146.8 ± 8.5 | 151.7 ± 13.0 | 0.077 | |||||
| UD5(Gy) | 195.4 ± 13.8 | 194.2 ± 9.4 | 0.69 | |||||
| Day 30 | RV100(ml) | 0.97 ± 0.69 | 1.00 ± 0.71 | 0.78 | ||||
| RV150(ml) | 0.07 ± 0.09 | 0.11 ± 24.3 | 0.34 | |||||
| UD90(Gy) | 165.4 ± 19.4 | 154.6 ± 24.3 | 0.056 | |||||
| UD5(Gy) | 251.7 ± 28.3 | 246.4 ± 31.3 | 0.48 | |||||
| Kaneda [16] | 76 | Day 30 | RV100(ml) | 0.44 ± 0.27 | 0.61 ± 0.35 | 0.03 | + | |
| RD2 cc(%) | 64.6 ± 10.4 | 68.1 ± 12.3 | 0.18 | |||||
| Major [17] | 205 | Day28 | UDmax(%) | 154 ± 14 | 138 ± 14 | <0.05 | - | |
| UD0.1 cc(Gy) | 203 ± 8 | 183 ± 9 | <0.05 | - | ||||
| UD10(%) | 135 ± 7 | 125 ± 8 | <0.05 | - | ||||
| UD30(%) | 128 ± 6 | 119 ± 7 | <0.05 | - | ||||
| RDmax(%) | 115 ± 19 | 101 ± 25 | <0.05 | - | ||||
| RD2 cc(Gy) | 98 ± 15 | 82 ± 17 | <0.05 | - | ||||
| RD0.1 cc(Gy) | 145 ± 18 | 127 ± 25 | <0.05 | - | ||||
| RD10(%) | 88 ± 11 | 75 ± 15 | <0.05 | - | ||||
| Laimonas [23] | 186 | Day 0 | UD90(Gy) | 120.5 ± 15.4 | 112.6 ± 12.2 | <0.001 | - | |
| UD30(Gy) | 193.2 ± 5.9 | 198.2 ± 6.8 | <0.001 | + | ||||
| UD10(Gy) | 201.3 ± 7.7 | 206.4 ± 7.7 | <0.001 | + | ||||
| RV100(ml) | 0.15 ± 0.1 | 0.26 ± 0.2 | <0.001 | + | ||||
| Day 30 | UD90(Gy) | 136.3 ± 20.2 | 139.8 ± 25.4 | 0.317 | ||||
| UD30(Gy) | 197.4 ± 19.5 | 218.6 ± 24.1 | 0.001 | + | ||||
| UD10(Gy) | 212.3 ± 24.0 | 234.3 ± 31.9 | <0.001 | + | ||||
| RV100(ml) | 0.3 ± 0.3 | 0.6 ± 0.47 | <0.001 | + |
Values are given as mean ± SD
if SD is available, U: urethral volume
R: rectal volume
n: number of cases
U: urethral volume
R: rectal volume
Operation time
Almost all researchers agreed that IBCL implant would take a longer operation time [10-15]. Hirose et al. did not show a significant difference between the operation time of the IBCL group and the LS group, but the number of patients in their study was small [14]. The findings of the literature search are summarized in Table 4.
Tab.4. Operation time
| Study(ref) | n | Parament | IBCL | LS | P | Advantage |
|---|---|---|---|---|---|---|
| Ishiyama [10] | 854 | Operation time(min) | 74.5 ± 23.7 | 64.2 ± 20.8 | <0.001 | - |
| Anesthesia time(min) | 103.4 ± 27.4 | 89.9 ± 25.0 | <0.001 | - | ||
| Zauls [11] | 91 | Operation time(min) | 94.2 ± 20.3 | 85.3 ± 20.4 | 0.022 | - |
| Ishiyama [12] | 140 | Operation time(min) | 57 ± 15 | 50 ± 13 | <0.001 | - |
| Inada [13] | 74 | Operation time(min) | 50.5 ± 12.6 | 43.7 ± 9.0 | 0.011 | - |
| Hirose [14] | 62 | Operation time(min)(PI) | 105 ± 17.7 | 100 ± 28.3 | 0.765 | |
| Operation time(min)(PI+EBRT) | 130 ± 13.0 | 110 ± 22.0 | 0.097 | |||
| Katayama [15] | 64 | Operation time(min) | 108.7 ± 16.6 | 102.0 ± 15.2 | 0.13 | |
| Operation time/seed(min) | 1.31 ± 0.21 | 1.13 ± 0.21 | 0.003 | - |
Values are given as mean ± SD, if SD is available, n: number of cases
Discussion
About target area volume dosimetric, stranded seeds had no significant disadvantage in dosimetry compared with LS. Actually, stranded seeds can have dosimetric advantages in maintaining the dose homogeneity in the target area. Due to the characteristics of interconnection, stranded seeds can help to achieve dose coverage in the periphery of target area as close as possible to the prescribed dose. In terms of tissue oedema, Saibishkumar et al. [24] speculated that the oedema caused by the local displacement and rotation of loose seeds was significantly more severe than that caused by stranded seeds, or the tissue stimulation caused by stranded seeds was smaller than that caused by loose seeds, leading to lighter tissue oedema and a higher dose of stranded seeds. The hypothesis of Saibishkumar et al. is interesting, but it remains to be further verified by more studies. The results of Pinkawa et al. [25] showed significantly increased dose to the target and rectum by every key parameter accompanied with the relief of postoperative tissue oedema. This indicated that an area of a few millimeters anterior to the rectal wall and to the urethra should be avoided in the initial treatment plan to reduce the risk of complications.
In terms of biochemical failure rate, given the inconsistent evidence in the literature, the logical conclusion is that either seed format can provide excellent results, or the bNED rate is related to dosimetric parameters. According to Hinnen et al. [27], D90 values decreased from intra-operative stage to postoperative stage, and from 184 to 153 Gy for stranded seeds and from 183 to 170 Gy for loose seeds. Dose reduction was significantly greater (p<0.001) for stranded seeds, and bNED showed a significant difference between LS and SS groups. However, in the study of Herbert et al. [18], the SS group had higher D90 compared with the LS group. Nevertheless, this does not mean that the two formats are interchangeable for a single operation, because the subtle differences in planning and technique may lead to noticeably different outcomes with loose versus stranded seeds [18]. In terms of seed migration, based on the published studies, the SS technique has significant advantages in reducing seed migration and local displacement. It may help to reduce the total number of seeds required since a high number of extra-prostatic seeds are not needed for a very generous treatment [9,20], which also reduces the risk of serious complications such as pulmonary embolism. In terms of OARs, SS and IBCL should be superior to LS, but some studies did not show the same results. However, there is no strong evidence indicating that seed link can reduce the incidence of complications. The reason may be that the dose to the organs at risk is also dependent on the quality of implants and the experience of the operator. In terms of operation time, IBCL can prolong operation time and anesthesia time, but sometimes the effect may not be significant. In summary, a high number of extra-prostatic seeds and large treatment margins can be the reasons why there is no significant discrepancy in the dosimetric parameters between SS and LS groups in some studies [32]. The study of Kudchadker et al. [20] on total implanted activity may confirm this hypothesis. The difference in dosimetric results among different studies may be caused by different imaging techniques used in different studies, and the difference between preplanned and real-time intraoperative techniques. For example, the study of Pinkawa et al.[33] demonstrated that the geometry between Transrectal Ultrasonography (TRUS) and CT/MRI was not always consistent. Differences in the levels of technological sophistication among different centers may also contribute to these differences. Combined with the study of a biochemical failure rate, it is indicated that SS has no disadvantage compared with loose seeds. Almost all studies focus on dosimetric parameters, and only a few studies followed up bNED and acute toxicity. So, it cannot be confirmed that the dosimetric superiority of SS will lead to significant clinical benefits. If possible, more studies shall be done to follow up bNED and acute toxicity, so as to determine whether dosimetric superiority leads to clinical benefits.
Conclusion
Due to its connection characteristics, SS can have latent dosimetric advantages in comparison with LS, especially in terms of dose homogeneity and coverage of peripheral target area. SS can also reduce the dose to OARs. SS has significant advantages in reducing seeds migration, local displacement and related complications. The increase of operation time is only seen in IBCL, but with the progression of technology, this issue will become less significant.
Acknowledgement
There are no conflicts of interest.
References
- Cooperberg MR, Lubeck DP, Mehta SS, Caroll PR. Time trends in clinical risk stratification for prostate cancer: Implications for outcomes (data from capsure). J Urol. 2003;170:S21-S27.
- Sylvester JE, Grimm PD, Blasko JC. 15-year biochemical relapse free survival in clinical stage t1-t3 prostate cancer following combined external beam radiotherapy and brachytherapy; seattle experience. Int J Radiat Oncol Biol Phys. 2007;67:57-64.
- Potters L, Morgenstern C, Calugaru E. 12-year outcomes following permanent prostate brachytherapy in patients with clinically localized prostate cancer. J Urol. 2005;173:1562-1566.
- Stock R G, Cahlon O, Cesaretti J A. Combined modality treatment in the management of high-risk prostate cancer. Intern J Radiat Oncol Biol Phys. 2004;59:1352-1359.
- Stock RG, Cesaretti JA, Stone NN. Disease-specific survival following the brachytherapy management of prostate cancer. Intern J Radiat Oncol Bio, Phys. 2005;64:810-816.
- Merrick GS, Butler WM, Wallner KE. Variability of prostate brachytherapy preimplant dosimetry: a multi-institutional analysis. Brachytherap. 2005;4:241-251.
- Marshall DT. Options and recent advances in permanent brachytherapy for prostate cancer. Canad J Urol. 2007;14 Suppl 1:28-31.
- Stock RG, Stone NN, Wesson MF. A modified technique allowing interactive ultrasound-guided three-dimensional transperineal prostate implantation. Intern J Radiat Oncol Biol Phys. 1995;32:219-225.
- Reed DR, Wallner KE, Merrick GS. A prospective randomized comparison of stranded vs. Loose 125i seeds for prostate brachytherapy. Brachytherap. 2007;6:129-134.
- Ishiyama H, Satoh T, Yorozu A. Multi-institutional retrospective analysis of learning curves on dosimetry and operation time before and after introduction of intraoperatively built custom-linked seeds in prostate brachytherapy. J Radiat Res. 2016;57:68-74.
- Zauls AJ, Ashenafi MS, Onicescu G. Comparison of intraoperatively built custom linked seeds versus loose seed gun applicator technique using real-time intraoperative planning for permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2011;81:1010-1016.
- Ishiyama H, Satoh T, Kawakami S. A prospective quasi-randomized comparison of intraoperatively built custom linked seeds versus loose seeds for prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2013;87:S347-S348.
- Masahiro I, Masaki Y, Takafumi M. Dosimetry advantages of intraoperatively built custom-linked seeds compared with loose seeds in permanent prostate brachytherapy. J Contemp Brachytherap. 2017;5:410-417.
- Hirose K, Aoki M, Sato M. Analysis of the relationship between prescribed dose and dosimetric advantage of real-time intraoperatively built custom-linked seeds in iodine-125 prostate brachytherapy. Radiat Oncol. 2017;12:192.
- Katayama N, Takemoto M, Takamoto A. Comparison of implant quality between intraoperatively built custom-linked seeds and loose seeds in permanent prostate brachytherapy using sector analysis. J Radiat Res. 2016;57:393-399.
- Tomoya K, Toshio O, Masanori S. Plan reproducibility of intraoperatively custom-built linked seeds compared to loose seeds for prostate brachytherapy. J Contemp Brachytherap. 2018;10:291-296.
- Major T, Agoston P, Fröhlich G. Loose versus stranded seeds in permanent prostate brachytherapy: Dosimetric comparison of intraoperative plans. Physica Medica 2014;30:909-913.
- Herbert C, Morris W J, Hamm J. The effect of loose versus stranded seeds on biochemical no evidence of disease in patients with carcinoma of the prostate treated with iodine-125 brachytherapy. Brachytherapy. 2011;10:442-448.
- Saibishkumar EP, Borg J, Yeung I. Sequential comparison of seed loss and prostate dosimetry of stranded seeds with loose seeds in 125i permanent implant for low-risk prostate cancer. Intern J Radiat Oncol Biol Phys. 2009;73:61-68.
- Kudchadker RJ, Swanson DA, Kuban A. Is a loose-seed nomogram still valid for prostate brachytherapy in a stranded-seed era? Intern J Radiat Oncol Biol Phys. 2008;72:623-627.
- Lee WR, DeGuzman AF, Tomlinson SK. Radioactive sources embedded in suture are associated with improved postimplant dosimetry in men treated with prostate brachytherapy. Radiotherap Oncol. 2002;65:123-127.
- Kaplan ID, Meskell MP, Marshal L. A comparison of the precision of seeds deposited as loose seeds versus suture embedded seeds: A randomized trial. Brachytherapy .2004;3:7-9.
- Laimonas J, Arturas I, Elona J. Comparison of implant quality between loose and intra-operatively linked iodine-125 seeds in prostate cancer brachytherapy. J Radiat Res. 2012;53:439-446.
- Saibishkumar EP, Borg J, Yeung I. Loose seeds vs. Stranded seeds: A comparison of critical organ dosimetry and acute toxicity in 125i permanent implant for low-risk prostate cancer. Brachytherapy. 2008;7:200-205.
- Pinkawa M, Asadpour B, Gagel B. Evaluation of source displacement and dose–volume changes after permanent prostate brachytherapy with stranded seeds. Radiotherap Oncol. 2007;84:190-196.
- Mclaughlin P, Narayana V, Pan C. Comparison of day 0 and day 14 dosimetry for permanent prostate implants using stranded seeds. Intern J Radiat Oncol Biol Phys. 2006;64:144-150.
- Hinnen KA, Moerland MA, Battermann JJ. Loose seeds versus stranded seeds in i-125 prostate brachytherapy: Differences in clinical outcome. Radiotherap Oncol. 2010;96:30-33.
- Merrell KW, Davis BJ, Goulet CC. Reducing seed migration to near zero with stranded-seed implants: Comparison of seed migration rates to the chest in 1000 permanent prostate brachytherapy patients undergoing implants with loose or stranded seeds. Brachytherap. 2019;18:306-312.
- Birckhead BJ, Fossum CC, Deufel CL. Stranded seed displacement, migration, and loss after permanent prostate brachytherapy as estimated by day 0 fluoroscopy and 4-month postimplant pelvic x-ray. Brachytherap. 2016;15:714-721.
- Vassiliev O N, Kudchadker R J, Swanson D A, et al. Displacement of periurethral stranded seeds and its dosimetric consequences in prostate brachytherapy. Brachytherap. 2011;10:401-408.
- Al-Qaisieh B, Carey B, Dan A. The use of linked seeds eliminates lung embolization following permanent seed implantation for prostate cancer. Int J Radiat Oncol Biol Phys. 2004;59:397-399.
- Merrick GS, Butler WM. Modified uniform seed loading for prostate brachytherapy: Rationale, design, and evaluation. Tech Urol. 2000;6:78-84.
- Pinkawa M, Asadpour B, Piroth MD. Rectal dosimetry following prostate brachytherapy with stranded seeds – comparison of transrectal ultrasound intra-operative planning (day 0) and computed tomography-postplanning (day 1 vs. Day 30) with special focus on sources placed close to the rectal. Radiotherap Oncol. 2009;91:207-212.

