Research Article - Onkologia i Radioterapia ( 2021) Volume 15, Issue 11
A study on pattern of locoregional recurrence and its relation to the irradiated volume in patients with carcinoma breast receiving adjuvant radiotherapy
Greeshma KE, Geetha Muttath*, Nabeel Yahia, Suja CA, Resmi K Bharathan and Arun KumarGeetha Muttath, Department of Radiation Oncology, Malabar Cancer Centre, Thalassery Kannur, PIN 670103, Kerala, India, Email: greethamkel234@gmail.com
Received: 01-Nov-2021 Accepted: 22-Nov-2021 Published: 26-Nov-2021
Abstract
Aims and objectives: Loco Regional Recurrences (LRR) can occur in up to 5%- 15% of patients with breast cancer despite appropriate treatment and this can potentially be a harbinger for subsequent systemic relapse. We analysed the incidence and patterns of LRR in breast cancer patients who received adjuvant radiotherapy from January 2015 to December 2018 at our centre.
Methodology: The planning CT scans and the imaging done at the time of recurrences were superimposed to find out if the recurrences are inside or outside the irradiated volume.
Results: After a median follow up of 37.5 months (18 m-52 m), 12 out of the 517 patients irradiated had LRR (2.3%). Median disease free interval was 14.5 m (1 m-52 m). 58.3% of patients had N2 or above nodal stage. 66.7% had Invasive carcinoma Gr2. Lympho vascular emboli was noted in 58.3%, perineural invasion in 33.3% and extra capsular extension in 58.3% of patients. Most common site of LRR was SCF (n=7) followed by CW (n=5). 91.7% of patients had Infield recurrence. Outside field recurrence occurred in 5 patients (41.7%), all of which occurred in the Internal Mammary Nodes (IMN). 50% of patients (6/12) had associated systemic relapse.
Conclusions: The rate of LRR among patients with breast cancer after adjuvant radiotherapy is low in our experience. 41.7% of all LRR involved IMN either in isolation or along with other sites. This highlights the importance of IMN as a site of LRR and hence the need to address the ipsilateral IMN in all patients receiving adjuvant radiotherapy for advanced breast cancer.
Keywords
breast cancer, loco regional recurrence, radiotherapy
Introduction
Breast cancer is the most common cancer in India with an incidence of 13.5% in the year 2020 [1]. Regional irradiation is an important strategy in the management of breast cancer as it reduces Loco Regional Recurrences (LRR) and thereby breast cancer related mortality. The inclusion of regional nodes in the irradiated volume improves the Disease Free Survival (DFS) when compared to whole breast irradiation or chest wall radiation alone. Post mastectomy irradiation in locally advanced breast cancer reduces LRR, overall recurrence and improve overall survival [2, 3]. The benefit of regional irradiation for patients with involved axillary nodes has been shown in metaanalysis to reduce LRR, overall recurrence and improve overall survival [4].
Despite treatment with adjuvant radiotherapy after mastectomy or Breast Conserving Surgery (BCS), LRR occur in approximately 5%-15% of patients [3,5-8]. The most common site of recurrence after adjuvant radiotherapy is the ipsilateral breast or the chest wall, comprising 60%-95% of all LRR [9,10]. Loco regional recurrences are predictor of an increased risk of concurrent or subsequent systemic relapse [11,12]. Early recurrences, especially within the first 2 years after primary treatment have a worse prognosis [13].
Mapping of patterns of LRR with respect to the presently accepted contouring consensus guidelines like European Society for Radiotherapy and Oncology (ESTRO) [14-15] and that of the Radiation Therapy Oncology Group (RTOG) [16] shows that these guidelines successfully encompass most areas of LRR. In the present study we analysed the incidence and pattern of LRR with respect to the irradiated volume in patients who received adjuvant radiotherapy for breast cancer at our centre.
Materials and Methods
We have done a retrospective study of patients who received adjuvant radiotherapy for breast cancer between January 2015 and December 2018 at our centre. The study was approved by the institutional Review board. All patients who received adjuvant radiotherapy after mastectomy as well as breast conservation surgery were included. Patients who received incomplete radiotherapy were excluded from the study. The indications for adjuvant radiotherapy included T stage of T3 or above, node positive disease and breast conservation surgery.
All patients who were planned for adjuvant radiotherapy underwent a plain CT scan with a slice thickness of 5mm after immobilisation on a dedicated CT simulator (Optima GE). At our centre, conventional 2D planning technique is used to delineate the radiation field for post mastectomy radiotherapy. Chest wall and supraclavicular fossa are treated for patients with T3 or above and for node positive patients. Medial and lateral tangents are used for treating the chest wall and a matched anterior field is used for the ipsilateral supraclavicular fossa. Entire axilla is included in the treatment field when there was evidence of extra capsular extension in the nodes in postoperative specimens. Internal Mammary Nodes (IMN) is not routinely included in the treatment volume and is irradiated when there was clinical or radiological evidence of positive IMN prior to treatment. A radiation dose of 40Gy/15 daily fractions, 5 days a week is prescribed.
For adjuvant radiotherapy after BCS, Three Dimensional Conformal Radiotherapy (3DCRT) is used at our centre. Contouring guidelines recommended by Radiation Therapy Oncology Group (RTOG) are followed for delineating the clinical target volumes [16]. The target volume includes the entire breast to a dose of 40Gy/15fr followed by boost RT 10Gy/5fr to the tumour bed which includes post-operative cavity with 1.5 cm expansion, including the surgical clips and post-operative scar. Breast alone is treated for T2N0 disease. Supraclavicular fossa is included in the irradiation volume in patients with T3 and T4 disease, node positive disease and for patients after neoadjuvant chemotherapy. Axilla is treated if there was evidence of extra capsular extension.
The case records of all patients who received adjuvant radiotherapy for carcinoma breast were reviewed. The demographic details, histopathological details, and hormonal status of all patients were recorded. The date of diagnosis of recurrence, the site of recurrence and the associated systemic recurrences if any, were also recorded.
The contrast enhanced CT scans done at the time of detection of recurrence were collected for those patients with LRR and were registered with planning CT scans done at the time of radiation planning to see if the sites of recurrence were inside the irradiated volume, outside the irradiated volume or at the margin of irradiated volume and recorded as infield, out of field and marginal recurrences respectively.
For post mastectomy patients, the planning scans with the laser points representing the field borders were transferred to the Varian Eclipse Version 13.6 treatment planning system and radiation fields and treated volume were recreated with the same field parameters as used for treating the patient. For those patients after BCS, the planning scans were available in the Varian Eclipse planning system. The diagnostic scan taken at the time of recurrence was registered with the planning scans and site of recurrence was contoured to see if the site of recurrence is infield, out of field or marginal.
The number (%) of patients with LRR among post mastectomy patients, patients after breast conservation surgery and both the groups combined were recorded. We also looked into whether any demographic or histopathological characteristics common among patients with LRR were present.
Statistical analysis
The quantitative variables were summarized using descriptive statistics and qualitative variables were summarized using number and percentage. Data were analysed using IBM SPSS statistical software, version 20.0 (SPSS, Armonk, NY:IBM Corp).
Results
517 patients received adjuvant radiotherapy for carcinoma of the breast at our centre from December 2015 to January 2018. 320 (61.9%) patients had undergone Modified Radical Mastectomy (MRM) and 197 (38.1%) patients had undergone Breast Conservation Surgery (BCS). Twelve patients (12/517, 2.3%) developed LRR during the period. Among these, 10 (83.3%) patients received post MRM RT and two patients (16.6%) post BCS RT.
Most common site of LRR was Supra Clavicular Fossa (SCF) (n=7; 58%) followed by chest wall (n=5; 41.6%), and axilla (n=2; 16.6%). More than one site of LRR was noted for four (33.3%) patients. All of them had recurrences in SCF and IMN. Two patients had LRR in the chest wall in addition, and one patient had LRR in the axilla in addition to SCF and IMN. The incidence and pattern of LRR is given in Table 1.
| Sites of relapse | Total Number | % | PMRT | Post BCS RT |
|---|---|---|---|---|
| CW | 3 | 25 | 3 | 0 |
| SCF | 3 | 25 | 3 | 0 |
| Axilla | 1 | 8 | 1 | 0 |
| IMN | 1 | 8 | 1 | 0 |
| CW, SCF, IMN | 2 | 16 | 2 | 0 |
| SCF, IMN | 1 | 8 | 0 | 1 |
| SCF, AXILLA, IMN | 1 | 8 | 0 | 1 |
| Total | 12/517 | 2.30% | 10/319 (3.1%) | 2/197 (1%) |
Tab. 1. Incidence and pattern of loco regional recurrences
Relationship of site of LRR and the irradiated volume
Out of the 12 patients with LRR, 11(91.6%) patients had inside field recurrence. Five patients recurred outside the radiation field. All out of field recurrences occurred in the Internal Mammary Node (IMN) (41.7%). There were no marginal recurrences.
The site of LRR in each patient and its relation to the irradiated volume are detailed in Table 2.
| Case Number | In Field | Out of field | Associated systemic recurrence |
|---|---|---|---|
| 1 | SCF | - | NO |
| 2 | Axilla | - | NO |
| 3 | Chest wall | - | NO |
| 4 | SCF, Axilla | IMN | YES |
| 5 | Chest wall | - | YES |
| 6 | SCF | IMN | YES |
| 7 | SCF | - | NO |
| 8 | Chest wall | - | NO |
| 9 | Chest wall, SCF | IMN | YES |
| 10 | Chest wall, SCF | IMN | YES |
| 11 | SCF | - | NO |
| 12 | - | IMN | YES |
Tab. 2. Sites of loco regional recurrence in each patient and their relation to the irradiated volume
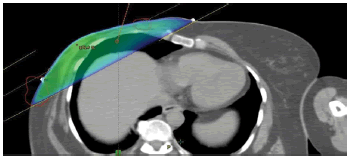
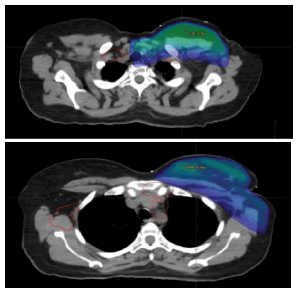
Planning CT scans were available for 7/12 patients. The CT scans taken after diagnosis of relapse were superimposed with CT scans taken during radiation planning for these patients. For the remaining 5 patients the sites of relapse were assessed clinically, radio logically and based on the records on the irradiated fields, the recurrence sites were categorised to be whether infield, out of field or marginal. Sample cases with infield recurrence are shown in Figure 1. The site of recurrence is contoured in red and superimposed onto the planning CT scan. The colour wash for 95% isodose is shown in blue colour. Sample case with infield recurrence and contralateral supraclavicular recurrence is shown in Figure 2. The same patient had out of field (Internal Mammary Node) recurrence.
Figure 1: Sample case with infield recurrence in the chest wall and axilla. The site of recurrence is contoured in red
Figure 2: Axial image of Sample Case With Infield (scf) recurrence. The site of recurrence is shown in red. The patient has contralateral supraclavicular lymph node and out of field (Internal Mammary Node) recurrence in addition
The median disease free interval (DFI) was 14.5 months (range 1 m- 52 m). Two patients relapsed one month after the completion of treatment. Majority (5/12) of patients had relapses between 10 and 15 months after completion of treatment. Three patients relapsed more than 47 months after completion of treatment.
Systemic relapse
Of the 517 patients, 28 patients had systemic relapse during the period (5.4%). Six out of the 28 (21.4%) patients had associated loco regional recurrence.
All four patients with multiple sites of LRR had associated systemic recurrence also. One patient each with isolated chest wall and IMN recurrence also had associated systemic relapse.
Clinical and Histological characteristics of patients with loco regional recurrence
The median age for patients with loco regional relapse was 43.5y (range 33y-54y). All patients had locally advanced disease. All except one patient had node positive disease, majority (7/12) having nodal stage of N2 or above. All twelve patients received chemotherapy.
Majority (8/12 patients) had invasive carcinoma grade 2 disease. Two patients had invasive carcinoma grade 3 disease, one patient had metaplastic carcinoma grade 3 disease and details on the grade of the disease were not available in one patient. Seven patients (58.3%) had evidence of lympho vascular emboli, four patients (33.3%) had evidence of perineural invasion and seven patients (58.3%) had extra capsular extension (Table 3).
| Median age | 43.5y (range 33y-54y) |
| Node positive disease | 11/12 (91.7%) |
| N2 or above | 7/12 (58.3%) |
| Histological grade | |
| Grade 2 | 8/12 (66.7%) |
| Grade 3 | 2/12 (16.7%) |
| Lympho vascular emboli | 7/12 (58.3%) |
| Perineural invasion | 4/12 (33.3%) |
| Extracapsular extension | 7/12 (58.3%) |
Tab. 3. Clinical and Histological characteristics of patients with LRR
Eight patients (66.7%) were Estrogen Receptor (ER) positive, six (50%) were Progesterone Receptor (PR) positive and six patients (50%) were Her 2 neu positive. Two patients had triple negative disease. Among the twelve patients, two patients were luminal A type, four patients were luminal
B Her 2 positive type, two patients were luminal B Her2 negative type, two patients were triple negative type and two patients were Her 2 positive type. The molecular subtypes of patients with LRR are given in Table 4.
| Case number | ER | PR | Her 2 neu | Ki 67 | Molecular classification |
|---|---|---|---|---|---|
| 1 | + | + | + | 30% | Luminal B, Her2+ |
| 2 | + | + | + | 10%-15% | Luminal B, Her2+ |
| 3 | + | + | - | 15%-20% | Luminal A |
| 4 | - | - | - | 60%-70% | TNBC |
| 5 | + | + | - | 40%-50% | Luminal B, Her2 (-) |
| 6 | + | - | - | 70%-80% | Luminal B, Her2(-) |
| 7 | + | + | + | Inconclusive | Luminal B, Her2 + |
| 8 | + | + | - | Inconclusive | Luminal A |
| 9 | - | - | + | 50%-55% | Her2+ like |
| 10 | - | - | - | Not done | TNBC |
| 11 | - | - | + | 20%-30% | Her2+ like |
| 12 | + | - | + | NA | Luminal B, Her2 + |
Tab. 4. Molecular subtypes of patients with loco regional recurrence
Discussion
The role of adjuvant radiotherapy in patients with carcinoma breast had been established in several large randomised trials and meta-analyses [4,10]. In breast cancer patients treated with mastectomy without PMRT, an overall LRR rate of 21.3% was demonstrated in the report from International Breast Cancer Study Group (IBCSG) after a median follow-up of 14.5 years [17]. Among these recurrences, the most common site of LRR was the chest wall (53%), followed by the supra/infraclavicular region (26%) and the axilla (13%). Tumour relapse at the internal mammary region was rarely reported (1%). Post mastectomy RT for patients with involved axillary nodes has been shown in meta-analysis to reduce LRR, overall recurrence and improve overall survival [4].
The EBCTCG analyses and the Danish Breast Cancer Studies have demonstrated that LRR occur in approximately 5%-15% of patients, despite treatment with adjuvant radiotherapy after mastectomy or Breast Conserving Surgery (BCS) [4-6,10]. The most common site of recurrence after adjuvant radiotherapy is the ipsilateral breast or the chest wall, comprising 60%-95% of all LRR [9, 10].
In our study, the LRR rate is 2.3% after a median follow up of 37.5 months (18-52 months). The lower rate of LRR noted may be due to the shorter follow up duration. The most common sites of recurrence were supraclavicular fossa (58%) and chest wall (41.6%) as demonstrated in other large trials.
The significant risk factors for LRR include young age, higher number of positive lymph nodes and higher grade of tumour [4, 18, 19]. The presence of perivascular invasion is also described to be a risk factor for LRR in some studies [20]. In our study, all patients with LRR had the majority of these risk factors. The patients had a median age of 43.5y. All patients had locally advanced disease and seven out of twelve patients (58.3%) had N2 or higher nodal stage. Eight patients had invasive carcinoma grade 2 disease (66.7%) and three patients had grade 3 disease (25%). Seven patients had evidence of lympho vascular emboli (58.3%). Four (33.3%) patients had evidence of perineural invasion and seven patients (58.3%) had extra capsular extension.
The immunohistochemical measurements for the expression of Estrogen Receptors (ER) and Progesterone Receptors (PR), human epidermal growth factor receptor 2 (HER2 neu), and the Ki-67 antigen are used to classify breast cancers and correlate well with genetically different breast cancer subtypes [21, 22], which are predictive of the risk of recurrence and outcome in addition to classic prognostic factors [23,24]. The prognosis is better for Luminal A subtype and it worsens in the order of luminal B, Her2 type and triple negative breast cancer.
In our study, two patients had luminal A type breast cancer, four patients were luminal B Her 2 positive type, two patients were luminal B, Her2 negative type, two patients were triple negative type and two patients were Her 2 positive type breast cancer. No definitive conclusion can be made from our study regarding the relation of the molecular subtype of breast cancer and the chance of recurrence, owing to the small number of patients with recurrence.
Loco regional recurrences are typically associated with an increased risk of concurrent or subsequent systemic relapse [11, 12]. In the EORTC 10801 and DBCG-82 TM analysis, out of the 1807 patients, 133 had local recurrence, the majority within 5 years after initial treatment [12]. An early LR is an indicator of a biologically aggressive tumour; early loco-regional relapse carries a poor prognosis and salvage treatment only cures a limited number of patients, whether treated by MRM or BCS originally. Early recurrences within the first 2 years after primary treatment seem to have a worse prognosis [25].
In our study, the majority of patients (58.3%) had relapsed less than 15 months after the completion of the treatment. Six out of the 12 patients with LRR (50%) had concurrent systemic relapse, of which three patients relapsed less than 15 months after the completion of the treatment. Four patients had multiple sites of LRR including IMN and one patient each had LRR in the chest wall alone and IMN alone.
A number of studies have evaluated the location of Regional Nodal Recurrences (RNR) in relation to the RTOG Breast Cancer Atlas [26-28] and the ESTRO Breast Cancer Atlas [29- 31] and have demonstrated that these guidelines, with some considerations, successfully encompassed most LRRs in patients undergoing contemporary management. In most of the studies, the majority of recurrences occurred inside the radiation field (in field) followed by out of field recurrences [32]. Marginal recurrences were less frequent. Eleven out of the twelve patients in our study (91.7%) had infield recurrences either alone or along with, out of field recurrence. There were no marginal recurrences.
All out of field recurrences were in the internal mammary nodes. The routine use of prophylactic IMN irradiation is controversial. Based on recent meta-analysis some guidelines recommend IMN irradiation in node positive cases [33]. In our study, the rate of LRR among patients with breast cancer after adjuvant radiotherapy is low but, five out of twelve (41.7%) patients had failed in the IMN. This highlights the importance of IMN as a site of LRR and hence the need to address the ipsilateral IMN in all patients receiving adjuvant radiotherapy for advanced breast cancer.
The limitations of our study are the small number of patients and short duration of follow up. Larger study including more number of patients with longer duration of follow up in a prospective manner is needed to arrive at a definitive conclusion regarding the need for routine use of IMN irradiation in patients with locally advance breast cancer.
Conclusion
The rate of loco regional recurrence among patients with breast cancer after adjuvant radiotherapy is low (12/517 patients, 2.3%) and is comparable to the available international data. Majority of the patients had infield recurrence, pointing to the biologically aggressive nature of the disease. All out of field recurrence occurred in internal mammary nodes highlighting the importance of inclusion of IMN in the irradiation volume.
Acknowledgement
We acknowledge the continuous support of Dr. Sameer EP in the compilation and finalisation of the manuscript. We thank Mrs Bindu Anil Kumar for the statistical support.
References
- The Global Cancer Observatory - March, 2021.
- Poortmans PM, Collette S, Kirkove C, Van Limbergen E, Budach V, et al. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med. 2015;373:317-327.
- McGale P, Correa C, Cutter D, Duane F, Ewertz M, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. The Lancet 2014;383:2127-2135.
- Darby S, McGale P, Correa C, Taylor C, Arriagada R, et al. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG): Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10 801 women in 17 randomized. trials. Lancet 2011;378:1707-1716.
- Christiansen P, Al-Suliman N, Bjerre K, Møller S. Recurrence pattern and prognosis in low-risk breast cancer patients–data from the DBCG 89-A programme. Acta oncologica 2008;47:691-703.
- Bartelink H, Maingon P, Poortmans P, Weltens C, Fourquet A, et al . Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol. 2015;16:47-56.
- Sedlmayer F, Sautter-Bihl ML, Budach W, Dunst J, Fastner G, et al. DEGRO practical guidelines: radiotherapy of breast cancer I. Strahlentherapie Und Onkologie. 2013;189:825-833.
- . Wahl AO, Rademaker A, Kiel KD, Jones EL, Marks LB,et al. Multi-institutional review of repeat irradiation of chest wall and breast for recurrent breast cancer. Int J Radiat Oncol Biol Phys. 2008;70:477-484.
- Nielsen HM, Overgaard M, Grau C, Jensen AR, Overgaard J. Loco-regional recurrence after mastectomy in high-risk breast cancer-risk and prognosis An analysis of patients from the DBCG 82 b&c randomization trials. Radiother Oncol. 2006;79:147-155.
- Bedwinek J. Natural history and management of isolated local-regional recurrence following mastectomy. In Seminars in Radiation Oncology 1994;4:260-269.
- Van Tienhoven G, Voogd AC, Peterse JL, Nielsen M, Andersen KW, et al. Prognosis after treatment for loco-regional recurrence after mastectomy or breast conserving therapy in two randomised trials (EORTC 10801 and DBCG-82TM). Eur J Cancer 1999;35:32-38.
- Wapnir IL, Anderson SJ, Mamounas EP, Geyer Jr CE, Jeong JH, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials J Clin Oncol. 2006;24:2028-2037.
- Offersen BV, Boersma LJ, Kirkove C, Hol S, Aznar MC,et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother Oncol. 2015;114:3-10.
- Offersen BV, Boersma LJ, Kirkove C, Hol S, Aznar MC,et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother Oncol. 2015;118:8-205.
- White JT, Arthur D, Buchholz T, MacDonald S,Marks L et al. Breast cancer atlas for radiation therapy planning:consensus definitions,accessed. 2017;73:944-951.
- Wallgren A, Bonetti M, Gelber RD, Goldhirsch A, Castiglione-Gertsch M, et al. Risk factors for locoregional recurrence among breast cancer patients: results from International Breast Cancer Study Group Trials I through VII. J Clin Oncol. 2003;21:1205-1213.
- Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thürlimann B, et al . Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009;20:1319-1329.
- Karlsson P, Cole BF, Chua BH, Price KN, Lindtner J,et al . Patterns and risk factors for locoregional failures after mastectomy for breast cancer: An International Breast Cancer Study Group report. Ann Oncol. 2012;23: 2852-2858
- Narayan P, Flynn J, Zhang Z, Gillespie EF, Mueller B, et al. Perineural invasion as a risk factor for locoregional recurrence of invasive breast cancer. Sci Rep. 2021;11:1-7
- Livasy CA, Karaca G, Nanda R, Tretiakova MS, Olopade OI, et al. Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Mod Pathol. 2006;19:264-271.
- . Park S, Koo JS, Kim MS, Park HS, Lee JS, et al. Characteristics and outcomes according to molecular subtypes of breast cancer as classified by a panel of four biomarkers using immunohistochemistry. Breast J. 2012;21:50-57.
- Lowery AJ, Kell MR, Glynn RW, Kerin MJ, Sweeney KJ. Locoregional recurrence after breast cancer surgery: a systematic review by receptor phenotype. Breast Cancer Res Treat. 2012;133:831-841.
- Ribelles N, Perez-Villa L, Jerez JM, Pajares B, Vicioso L, et al. Pattern of recurrence of early breast cancer is different according to intrinsic subtype and proliferation index. Breast Cancer Res 2013;15:1-6.
- Katz A, Strom EA, Buchholz TA, Thames HD, Smith CD, et al. Locoregional recurrence patterns after mastectomy and doxorubicin-based chemotherapy: implications for postoperative irradiation. J Clin Oncol. 2000;18:2817-2827.
- Brown LC, Diehn FE, Boughey JC, Childs SK, Park SS, et al. Delineation of supraclavicular target volumes in breast cancer radiation therapy. Int J Radiat Oncol Biol Phys. 2015;92:642-649.
- Gentile MS, Usman AA, Neuschler EI, Sathiaseelan V, Hayes JP, et al. Contouring guidelines for the axillary lymph nodes for the delivery of radiation therapy in breast cancer: evaluation of the RTOG breast cancer atlas Int J Radiat Oncol Biol Phys. 2015;93:257-265.
- Jing H, Wang SL, Li J, Xue M, Xiong ZK, et al. Mapping patterns of ipsilateral supraclavicular nodal metastases in breast cancer: Rethinking the clinical target volume for high-risk patients. Int J Radiat Oncol Biol Phys. 2015;93:268-276.
- Chang JS, Byun HK, Kim JW, Kim KH, Lee J, et al. Three-dimensional analysis of patterns of locoregional recurrence after treatment in breast cancer patients: Validation of the ESTRO consensus guideline on target volume. Radiother Oncol. 2017;122:24-29.
- Borm KJ, Voppichler J, Düsberg M, Oechsner M, Vag T, et al . FDG/PET-CT–Based Lymph Node Atlas in Breast Cancer Patients. Int J Radiat Oncol Biol Phys. 2019;103:574-582.
- DeSelm C, Yang TJ, Cahlon O, Tisnado J, Khan A, et al. A 3-dimensional mapping analysis of regional nodal recurrences in breast cancer. Int J Radiat Oncol Biol Phys. 2019;103:583-591.
- Beaton L, Nica L, Tyldesley S, Sek K, Ayre G, et al . PET/CT of breast cancer regional nodal recurrences: an evaluation of contouring atlases. Radiother Oncol. 2020;15:1.
- Laura Beaton, Luminita Nica, Scott Tyldesley, Kenny Sek, Gareth Ayre, et al. PET/CT of breast cancer regional nodal recurrences: an evaluation of contouring atlases Radiation Oncology .2020; 15:136.
- NCCN practice guidelines: breast cancer 2020.