Case Study - Onkologia i Radioterapia ( 2023) Volume 17, Issue 7
Colorectal cancer in young adults: Experience of the medical oncology department of fez
Youssef Elhaitmy*, Soukaina El Anssari, Lamiae Amaadour, Karima Oualla, Zineb Benbrahim, Samia Arifi and Nawfel MellasYoussef Elhaitmy, Department of Medical Oncology, University Hospital Centre Hassan II, City of Fez, Morocco, Tel: +212618171473, Email: youssef.elhaitmy@gmail.com
Received: 23-May-2023, Manuscript No. OAR-23-99285; Accepted: 01-Aug-2023, Pre QC No. OAR-23-99285 (PQ); Editor assigned: 16-Jun-2023, Pre QC No. OAR-23-99285 (PQ); Reviewed: 29-Jun-2023, QC No. OAR-23-99285 (Q); Revised: 25-Jul-2023, Manuscript No. OAR-23-99285 (R); Published: 07-Aug-2023
Abstract
Sporadic Colorectal Cancer (CRC) is usually diagnosed after the age of 60, and the current new surveillance guidelines only concern patients over the age of 50. However, an increased incidence of CRC in patients under 40 years of age has been described in many studies. This effect is attributed to molecular features and low suspicion of CRC in young symptomatic individuals. Besides that, knowledge of the differential diagnosis with CRC related to hereditary syndromes is important to provide treatment and adequate family screening. The evaluation of young adults must be very precise in order to avoid late diagnoses and consequently the increase in metastatic stages. The aim of our study is to describe the epidemiological characteristics as well as the differences in the criteria clinic-pathological and molecular aspects of young patients. This is a retrospective study of young patients with colorectal cancer aged <45 years and treated at the medical oncology department of Fez over a period from December 2009 to December 2020. Here, we review the changing epidemiology of CRC, tumour molecular features, and potential explanations for the increasing incidence of colorectal neoplasia in young individuals, which impact approaches to young patients with CRC treatment and prevention.
Keywords
colorectal cancer, young adults, epidemiology
Introduction
Colorectal Cancer (CRC) is the second digestive cancer in Morocco after gastric cancer [1]. Implementation of CRC screening in the mid-1990s has significantly decreased CRC incidence and mortality in individuals over the age of 50 [1, 2]. Paradoxically, a measurable increase in the incidence of CRC in individuals under the age of 50 has been observed, dating back to 1990 [3]. Although a number of hypotheses have been suggested, a clear risk factor has yet to be elucidated. Familial syndromes account for approximately 20% of early-onset CRC, and the majority of these tumours occur outside of known inherited syndromes (Lynch syndrome or polyposis). Young-onset CRC is more likely to occur in the distal colon or rectum, be poorly differentiated, have mucinous, and present at metastatic stages. Accurate screening in high-risk individuals and further investigation in symptomatic young adults may improve trends in early-onset CRC.
Case Presentation
This is a retrospective study which included 158 young patients treated at the medical oncology department of fez over a period from December 2009 to December 2020 suffering from colorectal cancer under the age of 45. To estimate the median survival, the Kaplan Meier method was used. The aim of this study is to report epidemiological, clinicopathological and molecular characteristics of young patients.
Results
The new European guidelines recommend screening for CRC from the age of 50. Recently, the American Cancer Society recommended starting screening at age 45. This approach was based on the increased incidence in younger subjects, modeling results and the assumption that screening the 45-49 age groups will have preventive effect as screening those 50 years and above.
In our study 158 (24%) patients were young, 16% had family history, and 11% were obese. Mucinous adenocarcinoma was present in 53%. 46% were at the metastatic stage (72 patients), of which 80% had a poorly differentiated character (Figure 1). MSI status was present in 62% at stage II. The mutated RAS status was 40%. 51% received tri-chemo-therapy versus 49% bichemo-therapy (Figure 2). The preferential site was in 52% the liver, 30% the lung and the bone in 16% [3]. However, young patients diagnosed with early-stage tumors had a significantly better prognosis compared to early-stage tumors in the older age group. The majority of young patients with CRC often received more aggressive treatment regimens. Median overall survival in young patients was 16 months.
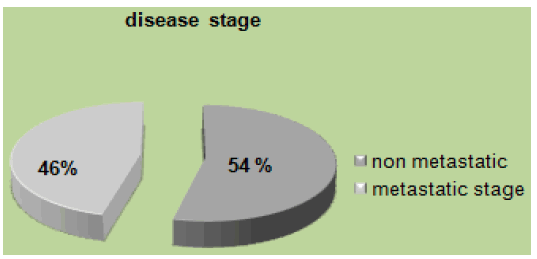
Figure 1: Disease stage
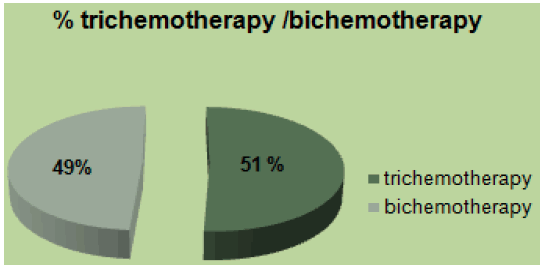
Figure 2: Percentage of tri-chemotherapy/bi-chemotherapy
Discussion
Since the implementation of routine screening for CRC in the 1990s, there have been significant and persistent overall decreases in both CRC incidence and mortality [1-6]. But, this trend concerns only individuals over the age of 50, while CRC incidence among young individuals under 50 years continues to rise with annual increases approaching, 1.7% for colon cancer and 2.6% in rectal cancer as measured from 2006 to 2015 [1]. The changing epidemiology of CRC is well illustrated by several studies analysing data from the US surveillance, epidemiology, and end results program demonstrating that CRC incidence increased by 1.4% per year among individuals aged under 50 years old while decreasing by 3.1% per year among those >50 years old [2,4,7-10]. Symptoms suggestive of CRC should be investigated with colonoscopy or, radiological imaging of the colon. Endoscopy is utilized both as a surveillance technique in patients with predisposing syndromes and as a diagnostic test in patients with symptoms suspicious for CRC. Colonoscopy has the advantage of affording biopsy with tissue diagnosis or polypectomy for small lesions (usually in surveillance), but can be technically difficult, particularly in the presence of obstructive symptoms when bowel preparation for the procedure is impossible. Colonic perforation and significant bleeding occur in approximately 0.2% of colonoscopies and small polyps may be missed even by an experienced endoscopist performing a complete examination [11]. Staging investigations should include Computed Tomography scan (CT) of the chest, abdomen, and pelvis with both liver and lung windows, to identify possible hepatic, pulmonary, or peritoneal metastases (Figures 3-5). Fluoro-deoxy-glucose-Positron Emission Tomography (FDG-PET) combined with CT has been used in adults for diagnosis, staging, and follow-up of CRC and is probably most useful for detecting distant metastatic disease. Its routine use is not currently recommended.
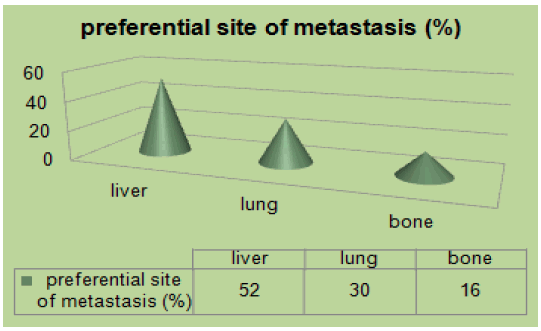
Figure 3: Preferential site of metastasis
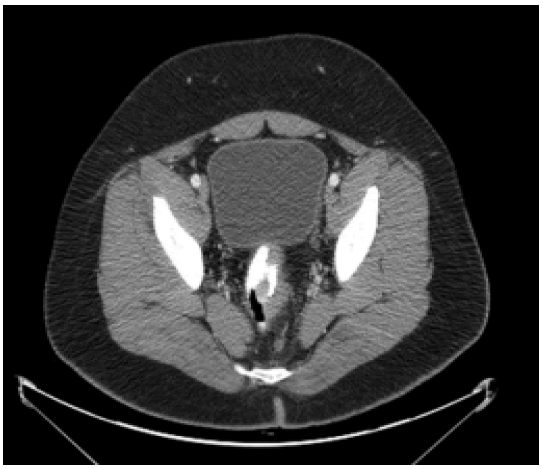
Figure 4: CT Scan of the pelvis of an 18-year-old patient demonstrating a low rectal carcinoma
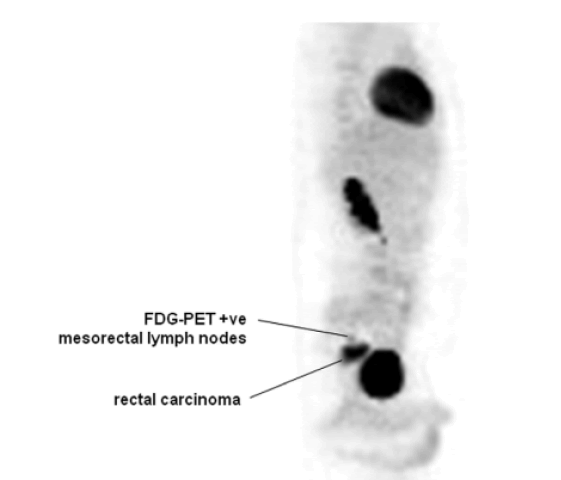
Figure 5: PET scan of the trunk of an 18-year-old patient demonstrating involvement of meso-rectal lymph nodes from a rectal carcinoma. It shows a PET scan of the same patient whose CT scan is depicted in Figure 4 and reveals the presence of regional lymph node metastases
Most series have suggested that young adults present with more aggressive disease histologically. Tumours are more often poorly differentiated, mucinous, and whose characteristics are often associated with adverse outcomes [1, 2, 12]. At a molecular level, CRC in young adults demonstrate a lower frequency of k-RAS mutations, and Loss of Heterozygosity (LOH) on chromosome 18 than older adults, while more tumours demonstrate MSI [13, 14]. It is important to consider the possibility of an underlying genetic predisposition to cancer in every individual affected with CRC at a younger age. Next-generation sequencing multigene panel genetic tests identify pathogenic germline variants in 16%- 20% of young CRC cases, involving genes associated with high and moderate penetrance cancer syndromes [15-17]. Lynch syndrome and familial adenomatous polyposis are the most prevalent syndromes, associated with early onset of colorectal neoplasia requiring colonoscopy beginning at age 20-25 and 10-12, respectively [16].Young adults are treated by adjuvant chemotherapy following surgical resection in the setting of stage III (node-positive) disease like older adults. Additionally, adjuvant chemotherapy is usually reserved for individuals with “high-risk” stage II disease (including those presenting with bowel perforation or obstruction; inadequate number of lymph nodes sampled; high-grade tumours; lymphatic, vascular, or perineural invasion). Although no studies have examined the benefit of adjuvant FOLFOX in young adults directly, the MOSAIC trial enrolled individuals as young as 18 years of age. Given the differences in tumour biology described above, and the higher frequency of MSI, it is unclear whether young adults would derive the same benefit from chemotherapy as their older counterparts.
The treatment of metastatic (stage IV) CRC has been revolutionized in recent years with the addition of several new chemotherapeutic agents, including irinotecan and oxaliplatin used in the metastatic setting. Although most cases are still incurable, a small subset of patients have durable survival (5 years-10 years) with aggressive chemotherapy and resection of limited metastases, especially when confined to the liver [18]. Survival in patients with unresecable disease has increased from a historical median of 6 months - 8 months to greater than 22 months in some series [19]. Details of chemotherapeutic regimens are beyond the scope of this review, but most such patients will benefit from treatment with multiple different agents throughout the course of their disease. Treatment often includes a combination of traditional cytotoxic chemotherapeutic agents with “targeted” molecular therapies. Although young adults have been eligible for the majority of trials evaluating these agents, there are very little published data specifically evaluating the efficacy of newer drugs in this patient population.
An issue extremely relevant to young adults undergoing adjuvant treatment of CRC is the effects of treatment on fertility. Adjuvant chemotherapy with 5-FU- based chemotherapy alone may have little influence on prospective female fertility; however, it is unknown whether newer agents, such as oxaliplatin, may contribute to irreversible premature ovarian failure [20]. Animal studies have suggested that oxaliplatin has at least moderate gonadotoxicity [21]. However, a study found that discussions surrounding post-treatment fertility are documented in less than 20% of women of childbearing age, and nearly 40% of young adults in this study had documented difficulty with pregnancy after treatment [22]. Additionally, it is felt that there is an inherent risk of infertility associated with surgery for CRC.
Conclusion
Early-onset colorectal carcinomas are a distinct clinical group characterized by aggressive evolution and poor cellular differentiation. Many factors may well explain this apparent epidemic among young people, namely and emerging lifestyle issues such as lack of exercise, obesity. A multidisciplinary approach is needed for managing these category patients and early referral to centres that are expert in the care of young adults with cancer will ensure the best achievable outcome. Whenever possible, managing these patients on clinical trials is preferable.
Conflicts of Interest
The authors declare no conflicts of interest
References
- Cancer Treatment Centers of America Cancer Treat Cent Am. 2022
- Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RG, et al. Colorectal cancer statistics, 2017. CA: Cancer J Clin. 2017;67:177-193.
- Murphy CC, Singal AG, Baron JA, Sandler RS. Decrease in incidence of young-onset colorectal cancer before recent increase. Gastroenterology. 2018;155:1716-1719.
- Siegel RL, Fedewa SA, Anderson WF, Miller KD, Ma J, et al. Colorectal cancer incidence patterns in the United States, 1974–2013. JNCI: J Natl Cancer Inst. 2017; 109:322.
- Bhandari A, Woodhouse M, Gupta S. Colorectal cancer is a leading cause of cancer incidence and mortality among adults younger than 50 years in the USA: a SEER-based analysis with comparison to other young-onset cancers. J Investig Med. 2017; 65:311-315.
- Sia CS, Paul E, Wale RJ, Lynch AC, Heriot AG, et al. No increase in colorectal cancer in patients under 50 years of age: a Victorian experience from the last decade. Colorectal Dis. 2014; 16:690-695.
- Yeo H, Betel D, Abelson JS, Zheng XE, Yantiss R, et al. Early-onset colorectal cancer is distinct from traditional colorectal cancer. Clin Colorectal Cancer. 2017; 16:293-299.
- Murphy CC, Lund JL, Sandler RS. Young-onset colorectal cancer: earlier diagnoses or increasing disease burden? Gastroenterology. 2017; 152:1809-1812.
- Murphy CC, Sanoff HK, Stitzenberg KB, Baron JA, Lund JL, et al. Patterns of sociodemographic and clinicopathologic characteristics of stages II and III colorectal cancer patients by age: examining potential mechanisms of young-onset disease. J cancer epidemiol. 2017; 2017.
- Siegel RL, Miller KD, Jemal A. Colorectal cancer mortality rates in adults aged 20 to 54 years in the United States, 1970-2014. Jama. 2017;318:572-574.
- Stevenson GW. Radiology and endoscopy in the pretreatment diagnostic management of colorectal cancer. Cancer. 1993;71:4198-4206.
- Bleyer A, Barr R, Hayes-Lattin B, Thomas D, Ellis C, et al. The distinctive biology of cancer in adolescents and young adults. Nat Rev Cancer. 2008; 8:288-298.
- Rajagopalan H, Nowak MA, Vogelstein B, Lengauer C. The significance of unstable chromosomes in colorectal cancer. Nat Rev Cancer. 2003; 3:695-701.
- Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005; 23:609-618.
[Google Scholar] [CrossRef]
- Pearlman R, Frankel WL, Swanson B, Zhao W, Yilmaz A, et al. Prevalence and spectrum of germline cancer susceptibility gene mutations among patients with early-onset colorectal cancer. JAMA oncol. 2017;3:464-471.
- Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, et al. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015; 110:223.
- Stoffel EM, Koeppe E, Everett J, Ulintz P, Kiel M, et al. Germline genetic features of young individuals with colorectal cancer. Gastroenterology. 2018; 154:897-905.
- Harmon KE, Ryan Jr JA, Biehl TR, Lee FT. Benefits and safety of hepatic resection for colorectal metastases. Am. J Surg. 1999; 177:402-404.
[Google Scholar ] [CrossRef]
- Tournigand C, André T, Achille E, Lledo G, Flesh M, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004; 22:229-237.
- Spanos CP, Mamopoulos A, Tsapas A, Syrakos T, Kiskinis D. Female fertility and colorectal cancer. Int J Colorectal Dis. 2008; 23:735-743.
- Marhhom E, Cohen I. Fertility preservation options for women with malignancies. Obstet gynecol surv. 2007; 62:58-72.
- Strong M, Peche W, Scaife C. Incidence of fertility counseling of women of child-bearing age before treatment for colorectal cancer. Am J Surg. 2007;194:765-768.

