Research Article - Onkologia i Radioterapia ( 2021) Volume 15, Issue 4
Comparison of patient controlled epidural analgesia with continuous epidural analgesia for postoperative pain control after surgeries for gynaecological cancers- a randomized controlled study
Jashma Chandveettil1*, Gnanam Sathyamurthy2 and Rajani Sundar22Department of Anaesthesiology G Kuppusamy Memorial Hospital, Coimbatore, India
Jashma Chandveettil, Department of anaesthesiology, Malabar Cancer Centre, Thalassery, Kerala. PIN - 670103, India, Email: jashmanizam@gmail.com
Received: 01-Apr-2021 Accepted: 21-Apr-2021 Published: 26-Apr-2021
Abstract
Introduction: Pain free recuperation is the basic right of every surgical patient. Postoperative pain has a deleterious effect on the short-term and long-term outcome of patients. In this study, we compared Patient Controlled Epidural Analgesia (PCEA) with background infusion with Continuous Epidural Analgesia (CEA) for quality of pain relief in postoperative patients with gynaecological cancers. Materials and methods: This was a randomized controlled trial conducted in a tertiary hospital in India. The patients with gynaecological cancers who underwent surgery with midline laparotomy were randomized into two groups. One group received CEA (Group-CEA) with ropivacaine and fentanyl at 6-10 ml/hour titrated for pain and the other group received CEA at a basal dose of 6ml/hour and bolus PCEA (Group-PCEA) for residual pain. The patients were studied for 36 hours after extubation. Primary outcome measured was the pain score using the Numerical Rating Scale (NRS) and secondary outcomes were morphine consumption, hemodynamic parameters, motor and sensory block and adverse effects. Results: The mean NRS pain scores up to 6 hours, 24 hours and 36 hours for Group-CEA and Group-PCEA were 0.23±0.39, 0.13±0.13 and 3.6±6.1 and 0.36±0.60, 0.19±0.26 and 5.7±6.1 respectively. The Differences in pain score and morphine consumption between CEA and PCEA groups at different time points namely up to 6th, 24th and 36th hours were calculated. The pain scores and morphine consumption in the CEA and PCEA groups was not statistically significant at any time points. Two patients in the Group-PCEA and none in the Group-CEA had motor block. The level of sensory block, effect on the hemodynamic parameters and adverse effects were comparable. Conclusion: In gynaecological cancer surgeries performed through midline laparotomy, both CEA and PCEA with background infusion provide effective analgesia with no significant difference in pain scores.
Keywords
patient controlled epidural analgesia, continuous epidural analgesia, postoperative pain gynaecological cancer
Introduction
Postoperative pain continues to be undertreated and results in a variety of unfavourable short- and long-term outcomes [1]. Pain has detrimental effects on postoperative recovery due to raised neuro-endocrine stress response and increased sympathetic stimulation [2]. This lead to negative nitrogen balance, protein catabolism and other adverse effects on patient health [3] leading to increased morbidity, longer hospital stay and significantly higher mortality [4] and chronic post-surgical pain [1].
With the exception of endometrial cancers, in gynaecological malignancies, Minimally Invasive Surgery (MIS) has not been accepted as the standard of care. In ovarian cancers, oncosurgeons to date utilize a midline incision sometimes extending from the xiphisternum to the pubic symphysis for cyto-reductive surgery. In carcinoma cervix, recent data have cast doubts on the role of MIS [5] prompting several surgeons to revert back to open surgery. This has put the onus of providing adequate pain relief to these patients on the surgical team with the anaesthesiologist in the forefront.
In a multimodality approach, epidural anaesthesia and analgesia play integral roles because of superior analgesia and favourable physiological effects compared to intravenous analgesia [1,2, 6-9]. It has been shown to improve the quality of patient recovery and reduce the incidence of serious postoperative morbidity both Continuous Epidural Analgesia (CEA) and Patient Controlled Epidural Analgesia (PCEA) are used widely [3].
There is paucity of studies comparing PCEA and CEA in gynaecological cancers and no such study has been reported from India. We wanted to compare the effectiveness of PCEA and CEA in gynaecological oncological surgeries. In this prospective randomized study we test our hypothesis that PCEA is as effective as CEA in pain control in postoperative patients after gynaecological oncological surgery.
Materials and Methods
This is a prospective randomized controlled study conducted at a tertiary hospital in India. The study protocol was approved by the Institutional Ethics Committee and the Scientific Committee (Supplementary Appendix 1: Study Protocol).
Patients with ASA I or ASA II status, undergoing cyto-reductive surgery for ovarian cancer, radical hysterectomy for cervical cancer and staging surgery endometrial cancers were included in the study. Obese patients with Body Mass Index (BMI) more than 35 kg/M2 were excluded. Written informed consent was obtained from all patients.
The primary outcome of the study was pain score, while secondary outcomes were morphine consumption, hemodynamic parameters, motor block and sensory block and adverse effects. To obtain a study power of 80% and a confidence level of 95%, sample size was calculated as 30 in each group. Randomisation was done by computer generated random numbers. The patients were allocated into two groups, those receiving CEA (Group-CEA) and those receiving PCEA (Group-PCEA) using computer generated random numbers.
Patients in Group-PCEA were preoperatively shown the PCEA pump and educated how to use the bolus button by the anaesthesiologists. All patients were given general anaesthesia with orotracheal intubation. In both the groups, anaesthesia was induced with thiopentone, fentanyl and vecuronium and was maintained with isoflurane with intermittent doses of fentanyl and vecuronium as required. In both groups, prior to induction, epidural catheter was inserted using Landmark technique under local anaesthesia in L1-L2 or L2-L3 interspaces. All patients underwent surgery through open surgical approach using midline laparotomy incision starting from the pubic symphysis and extending above the umbilicus depending on the extent of surgery.
CEA Group
Intra-operatively, background epidural infusion of 0.1% ropivacaine with fentanyl 2 mcg/ml at 6 ml/hr was started with an infusion pump after a bolus dose of 6ml postoperatively, background infusion was continued at 6 ml/hour in the Group CEA, and the rate was increased up to 10 ml/hour by the staff nurse depending on the pain score.
PCEA Group
In PCEA Group Intra-operatively, back ground epidural infusion of 0.1% ropivacaine with fentanyl 2 mcg/ml at 6 ml/hr was started with an infusion pump after a bolus dose of 6 ml. In the postoperative period, epidural infusion was continued at the same rate using PCEA pumps and any residual pain was managed with bolus PCEA administration. The bolus dose was set as 4 ml with a lock-out interval of 30 minutes, bringing to a dose limit of 14 ml in an hour. The doses were calculated such that they did not exceed the toxic dose of ropivacaine [10, 11].
Assessment
Pain was assessed using Numerical Rating Scale (NRS). The NRS is a subjective measure in which individuals rate their pain on an eleven-point visual analog scale. The scale depicts numbers 0 (no pain at all) to 10 (worst imaginable pain). The patient is shown a visual scale and is asked to choose a number corresponding to the severity of pain. NRS has been validated as a simple tool for judgment of pain in Indian rural patients [12]. The sensory blockade was documented once in every four hours up to 36 hours.
Pain was assessed using NRS by anaesthesiologists or trained nurses hourly till 24 hours and at two hourly till 36 hours when the patient was awake. The pain score was recorded as zero if the patient was asleep. All patients were given intravenous paracetamol at 1000 mg IV at eight-hour intervals and switched over to tablet paracetamol 1000 mg once in eight hours after oral diet was started, usually on the 3rd or 4th postoperative day. The set goal for pain relief was less than 3 on the NRS. If pain remains after administration of epidural analgesia, one mg of morphine was administered intravenously as rescue analgesic in both the groups, if the pain score was 3 or more (in Group-PCEA rescue analgesia was used if the patient continued to feel pain during the lock-out interval of 30 minutes after PCEA bolus). The sensory blockade was assessed with ice cubes covered with gauze and the motor blockade using Modified Bromage Scale (0=none, able to move ankle joint and flex and extend knee joint; 1=partial, patient is able to move ankle joint and extend knees; 2=able to move ankle joint; 3=complete, patient is unable to move lower limbs).
Statistical Analysis
IBM Corp. Released 2011 IBM SPSS (Statistical Package for the Social Sciences) Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp was used for the analysis. The data were reported as mean with SD or the median, depending on their distribution. The quantitative variables were presented as number and percentage. The Shapiro-Wilk test was used to test the normality of pain score and morphine consumption in CEA and PCEA groups at 6th, 24th and 36th hours. The differences in quantitative variables between groups were assessed by means of the unpaired t-test. Comparison between groups was made by the non-parametric Mann-Whitney test. Chi-square test for association was used to assess differences in categorical variables between groups. Wilcoxon signed rank test was used to assess the variables. p-value of <0.05 using a two-tailed test was taken as being of significance for all statistical tests.
Results
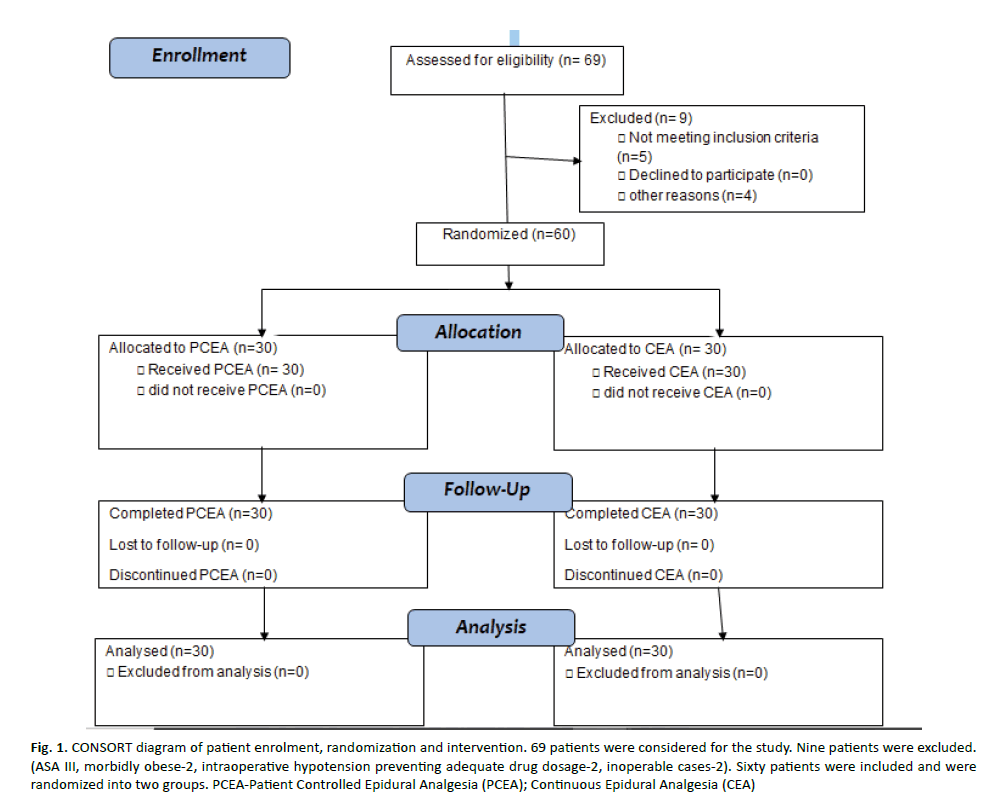
Sixty-nine patients were evaluated for the study. Nine patients were excluded. (ASA III-3, morbidly obese-2, intra-operative hypotension preventing adequate drug dosage-2, inoperable cases-2). Sixty patients were included and were randomized into two groups (Figure 1-CONSORT diagram). The baseline characteristics of age, weight and height were comparable in the two groups (Table 1). Age, height, weight, type of cancer, type of surgery and ASA status were comparable between the groups. Overall, 9 patients were diabetic, 12 were hypertensive and 3 were hypothyroid. Eleven patients had multiple comorbidities.
Figure 1: CO NSORT diagram of patient enrolment, randomization and intervention. 69 patients were considered for the study. Nine patients were excluded. (ASA III, morbidly obese-2, intraoperative hypotension preventing adequate drug dosage-2, inoperable cases-2). Sixty patients were included and were randomized into two groups. PCEA-Patient Controlled Epidural Analgesia (PCEA); Continuous Epidural Analgesia (CEA)
Tab. 1. Shows the demographic characteristics, ASA status, cancer diagnosis and the surgical procedure performed in the two groups of patients who underwent Continuous Epidural Analgesia (Group-CEA) or through a patient controlled device as Patient Controlled Epidural Analgesia (Group-PCEA)
| Patient factor | Group | Status | p-value |
|---|---|---|---|
| Age | Group-CEA | 52 (±10) years | 0.68 |
| Group-PCEA | 53 (±12) years | ||
| Average (combined) | 52 years | ||
| Height | Group-CEA | 153 cm | 0.08 |
| Group-PCEA | 156 cm | ||
| Average (combined) | 155 cm | ||
| Weight | Group-CEA | 61 (±10) Kg | 0.92 |
| Group-PCEA | 61 (±10) Kg | ||
| Average (combined) | 61 Kg | ||
| ASA status | Group-CEA | ASA I-22, | 0.248 |
| ASA II-8 | |||
| Group-PCEA | ASA I-18, | ||
| ASA II-12 | |||
| Cancer diagnosis | Group-CEA | Ca cervix-1 | 0.194 |
| Ca endometrium-15 | |||
| Ca ovary-14 | |||
| Group-PCEA | Ca cervix-5 | ||
| Ca endometrium-11 | |||
| Ca ovary-14 | |||
| Surgical procedure | Group-CEA | Staging laparotomy-24 | 0.368 |
| Interval cyto-reduction-5 | |||
| Wertheim’s hysterectomy-1 | |||
| Group-PCEA | Staging laparotomy-21 | ||
| Interval cyto-reduction-5 | |||
| Wertheim’s hysterectomy – 4 |
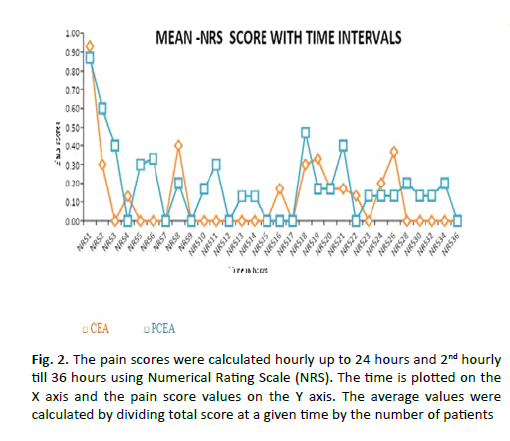
The mean NRS pain scores up to 6 hours, 24 hours and 36 hours for Group-CEA and Group-PCEA were 0.23 ± 0.39, 0.13 ± 0.13 and 3.6 ± 6.1 and 0.36 ± 0.60, 0.19 ± 0.26 and 5.7 ± 6.1 respectively (Table 2). The average pain scores between the two groups calculated up to 24 hours and up to 36 hours did not show significant difference (Figure 2). The pain score and morphine consumption in CEA and PCEA groups at 6th, 24th and 36th hours were not normally distributed (p=0.001). The difference in pain scores and morphine consumption upto 6th, 24th and 36th hours were not statistically significant between the two groups (Table 2).
Figure 2: The pain scores were calculated hourly up to 24 hours and 2nd hourly till 36 hours using Numerical Rating Scale (NRS). The time is plotted on the X axis and the pain score values on the Y axis. The average values were calculated by dividing total score at a given time by the number of patients
Tab. 2. Mean NRS scores and mean morphine consumption till 36 hours with respective p values and mann whitney u values are shown in the table. The NRS values were calculated by the formula, mean pain score=total NRS scores till the time of assessment/(number of patients X number of readings per patient till time of assessment). The mean morphine consumption was similarly calculated using the formula, mean morphine consumption=total morphine consumption till the time of assessment/(number of patients X number of readings per patient till time of assessment)
| Time in hours | Mean NRS scores (mean ± standard deviation) | Mann Whitney U | p value | |
|---|---|---|---|---|
| Group-CEA | Group-PCEA | |||
| 0-6 | 0.23 ± 0.39 | 0.36 ± 0.60 | 402 | 0.381 |
| 0-24 | 0.13 ± 0.13 | 0.195 ± 0.26 | 423 | 0.676 |
| 0-36 | 3.6 ± 6.1 | 5.7 ± 6.1 | 441 | 0.896 |
| Mean Morphine consumption in milligrams (mean ± standard deviation) | Mann Whitney U | P value | ||
| Group-CEA | Group-PCEA | |||
| 0-6 | 0.05 ± 0.09 | 0.12 ±0.20 | 397.5 | 0.353 |
| 0-24 | 0.03 ± 0.04 | 0.06 ± 0.09 | 398.5 | 0.419 |
| 0-36 | 0.97 ± 1.6 | 1.9 ± 2 | 401 | 0.446 |
Tab. 4. The number of patients in each group who achieved various sensory levels assessed at four hour intervals with respective p values is shown in the table
| Time of assessment | Group under study | Sensory level and number of patients who attained the particular level | |||||
|---|---|---|---|---|---|---|---|
| T8 | T10 | T12 | L1 | L2 | p value | ||
| 4th hour | Group-CEA | 10 | 7 | 0 | 4 | 0 | 0.228 |
| Group-PCEA | 19 | 17 | 1 | 1 | 2 | ||
| 8th hour | Group-CEA | 10 | 7 | 0 | 1 | 0 | 0.038 |
| Group-PCEA | 19 | 17 | 0 | 4 | 0 | ||
| 12th hour | Group-CEA | 10 | 7 | 1 | 1 | 2 | 0.435 |
| Group-PCEA | 19 | 17 | 0 | 1 | 0 | ||
| 16th hour | Group-CEA | 10 | 7 | 0 | 4 | 0 | 0.399 |
| Group-PCEA | 19 | 17 | 1 | 1 | 2 | ||
| 20th hour | Group-CEA | 10 | 7 | 0 | 1 | 0 | 0.001 |
| Group-PCEA | 19 | 17 | 0 | 4 | 0 | ||
| 24th hour | Group-CEA | 10 | 7 | 1 | 1 | 2 | 0.682 |
| Group-PCEA | 19 | 17 | 0 | 1 | 0 | ||
| 28th hour | Group-CEA | 10 | 7 | 0 | 4 | 0 | 0.703 |
| Group-PCEA | 19 | 17 | 1 | 1 | 2 | ||
| 32nd hour | Group-CEA | 10 | 7 | 0 | 1 | 0 | |
| Group-PCEA | 19 | 17 | 0 | 4 | 0 | 0.289 | |
| 36th hour | Group-CEA | 10 | 7 | 1 | 1 | 2 | 0.052 |
| Group-PCEA | 19 | 17 | 0 | 1 | 0 | ||
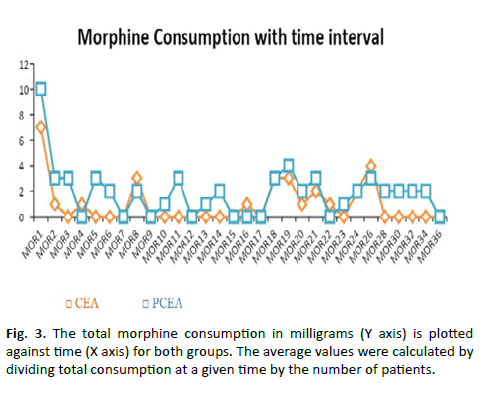
Hemodynamic variables (heart rate, systolic and diastolic Blood Pressure [BP] and respiratory rate) were compared between the groups and the results are shown in Table 3. At the 8th hour (p=0.038) and 20th hour (p=0.001), more subjects in Group-CEA had higher sensory level (T8) when compared to Group-PCEA (p=0.038) (Table 4). However, when the average was calculated for 36 hours, the difference was found to be statistically insignificant (p=0.093) (Figure 3).
Figure 3: The total morphine consumption in milligrams (Y axis) is plotted against time (X axis) for both groups. The average values were calculated by dividing total consumption at a given time by the number of patients.
Tab. 3. The hemodynamic variable in the two groups under study and the respective p values
| Variable | Group | Mean Value | p value |
|---|---|---|---|
| Mean Heart rate | Group-CEA | 79/minute | 0.527 |
| Group-PCEA | 82//minute | ||
| Mean Systolic BP | Group-CEA | 124 mm Hg | 0.898 |
| Group-PCEA | 123 mm Hg | ||
| Mean Diastolic BP | Group-CEA | 68 mm Hg | 0.991 |
| Group-PCEA | 68 mm Hg | ||
| Mean Respiratory rate | Group-CEA | 20/minute | 0.082 |
| Group-PCEA | 19/minute |
Two patients had motor blockades in the Group-PCEA, grade 1 in one patient and grade 3 in another patient. No patients in Group-CEA had motor block. In Group-PCEA, two patients had vomiting and one
Patient complained of nausea. There were no adverse effects reported in Group-CEA.
Discussion
Our study showed that PCEA with background infusion of ropivacaine and fentanyl for pain control in postoperative patients after surgery for cancer of ovary, endometrium and uterine cervix is comparable to CEA. Overall pain scores and morphine consumptions were low in both the groups showing that epidural analgesia is an effective method for pain control in postoperative patients with gynaecological cancers requiring open laparotomy irrespective of the method of infusion. The outcome from published studies comparing PCEA and CEA were heterogeneous in terms of analgesic efficiency, requirement of rescue analgesia and adverse effects [1,13-15].
In a study by Nightingale et al. [13]. In patients post colonic resection, lower pain scores were obtained in PCEA with background infusion compared to CEA [13]. Similar results were obtained in a study by Standl et al. [14] where the same cohort of patients were included for CEA and PCEA without background infusion at different points of time after the surgery, practically eliminating any chance of bias that would have come from variable individual pain threshold.
Suhaila N et al. [15] in a prospective, randomized study comparing the effectiveness of PCEA versus CEA in providing pain relief after gynaecological surgery found no significant difference in pain score, total amount of analgesics used, number of aesthetic interventions and patient satisfaction. They concluded that PCEA was comparable to CEA for pain relief after gynaecological surgery.
Van Samkar G et al. [16-19] in a retrospective cohort study compared PCEA with CEA in major thoracic and abdominal surgeries and found use of PCEA significantly reduced the number of patients requiring top-ups, while NRS scores did not differ between groups. There were no significant intergroup differences in NRS scores on Postoperative day 1 to 4. They concluded that PCEA can reduce frequency of top-ups and side effects compared to CEA.
A meta-analysis done by Christopher L. Wu et al. [1] has shown that mean pain scores at rest were less for Continuous Epidural Infusion (CEI) compared to PCEA. They concluded that CEI provided significantly superior analgesia (p<0.001) than PCEA for overall pain, pain at rest, and pain with activity. The authors also found that any form of epidural analgesia provides better pain control than patient controlled intravenous analgesia.
The different drugs, concentrations, rates of infusions, site of incisions and patient populations studied would have played a role in the variable outcomes in the studies done in different parts of the world. In our study, we calculated the amount of morphine consumption as a rescue analgesic as a surrogate to assess the quality of pain control by PCEA or CEA. The apprehension of sophisticated instruments like PCEA pumps and fear of self-injection of a drug could not be significantly eliminated by preoperative educational sessions on the pump usage especially in the elderly and those with lower educational background.
The hemodynamic parameters remained stable in both the groups indicating that there is no significant blockade of the autonomous system. Two patients in Group-PCEA had motor blocks while no patient had a motor block in Group-CEA. Though our incidence is small, this is in contrast with published literature which keeps those receiving PCEA at advantage with either same or decreased risk of motor block [15-19] with the small incidence; it is difficult to reach a definite conclusion though.
The sensory level achieved was significantly higher at 8th hour and 20th hour for Group-CEA. The differences were not statistically significant for the entire 36 hours. The higher rate of continuous infusion in some of these patients in Group-CEA probably explains the trend towards slightly higher levels of sensory block in these patients.
Limitations of the Study
The limitations of our study were [1] Doctors and Staff nurses monitoring the patients were not blinded [2] Activation of occlusion alarms is one problem that we came across in some patients in the PCEA arm of the study when administering the bolus doses of the drug via the narrow epidural catheter.
Strength of the Study
The complexity and duration of surgeries which may influence postoperative pain were mostly homogenous between the two groups.
Conclusion
In gynaecological oncological surgeries, postoperative pain relief with continuous epidural infusion and patient controlled epidural infusion is comparable. Both methods can be effectively used in patients after gynaecological cancer surgeries performed through midline laparotomy and the requirement of breakthrough pain relief with IV morphine is very low.
Conflict of Interest
Authors declare there is no conflict of interest.
References
- Christopher LW, Seth RC, Jeffrey MR, Andrew JR, Genevieve EC, et al. Efficacy of postoperative patient-controlled and continuous infusion epidural analgesia versus intravenous patient-controlled analgesia with opioids: a meta-analysis. Anaesthesiol. 2005;103:1079-1088.
- Wu CL, Hurley W. Acute Postoperative Pain. In: Miller RD, Eriksson LI, Fleisher LA, Wiener-Kronish JP, Young WL. Miller’s anaesthesia. 7th ed. United States of America: Natasha Andjelkovic; 2009;2757-2781.
- Susan MN. Benefit and outcome after epidural analgesia. Contin Educ Anaesth Crit Care Pain. 2004; 4:44-47.
- Ramsay MA. Acute postoperative pain management. Proc Bayl Univ Med Cent. 2000;13:244-247.
- Pedro TR, Michael F, Rene P, Lopez A, Vieira M, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018; 379:1895-1904.
- Salicath JH, Yeoh EC, Bennett MH. Epidural analgesia versus patient-controlled intravenous analgesia for pain following intra-abdominal surgery in adults. Cochrane Database Syst Rev. 2018;8:CD010434.
- Tucker K, Murray-Krezan C, Muller C, Rutledge TA. comparison of epidural analgesia and patient controlled intravenous analgesia on postoperative pain control and recovery parameters in women undergoing laparotomy for gynaecologic malignancy. Gynaecologic Oncol. 2015;137:82.
- Chen LM, Weinberg VK, Chen C, Bethan P, Chen L, et al. Perioperative outcomes comparing patient controlled epidural versus intravenous analgesia in gynaecologic oncology surgery. Gynaecologic Oncol. 2009;115:357-361.
- Viscusi ER. Patient-controlled drug delivery for acute postoperative pain management: a review of current and emerging technologies. Reg Anesth Pain Med. 2008;33:146-158.
- Kampe S, Weigand C, Kaufmann J, Klimek M, König DP, et al. Postoperative analgesia with no motor block by continuous epidural infusion of ropivacaine 0.1% and sufentanil after total hip replacement. Anesth Analg. 1999;89:395-398.
- Whiteside R, Jones D, Bignell S, Lang C, Lo SK. Epidural ropivacaine with fentanyl following major gynaecological surgery: the effect of volume and concentration on pain relief and motor impairment. Br J Anaesth. 2000;84:720-724.
- Mudgalkar N, Bele SD, Valsangkar S, Bodhare TN, Gorre M. Utility of numerical and visual analog scales for evaluating the post-operative pain in rural patients. Indian J Anaesth. 2012;56:553-557.
- Nightingale JJ, Knight MV, Higgins B, Dean T. Randomized, double-blind comparison of patient-controlled epidural infusion vs nurse-administered epidural infusion for postoperative analgesia in patients undergoing colonic resection. Br J Anaesth. 2007;98:380-384.
- Thomas S, Marc-Alexander B, Henning O, Stephan W, Martina S, et al. Patient-controlled epidural analgesia reduces analgesic requirements compared to continuous epidural infusion after major abdominal surgery. Can J Anesth. 2003;50:258-264.
- Suhaila N, Nurlia Y, Azmil Farid Z, Melvin K, Muhammad M, et al. Comparison between patient-controlled epidural analgesia and continuous epidural infusion for pain relief after gynaecological surgery. J Surg Acad. 2013;3:14-19.
- Van SG, Hermanns H, Lirk P, Hollmann MW, Stevens MF. Influence on number of top-ups after implementing patient controlled epidural analgesia: a cohort study. PLoS ONE. 2017;12:e0186225.
- Ferrante FM, Lu L, Jamison SB, Datta S. Patient-controlled epidural analgesia: demand dosing. Anesth Analg. 1991;73:547-552.
- Van der M, Halpern S, Joseph G. Patient-controlled epidural analgesia versus continuous infusion for labour analgesia: a meta-analysis. Br J Anaesth. 2002; 89: 459-465.
- Collis RE, Plaat FS, Morgan BM. Comparison of midwife top-ups, continuous infusion and patient-controlled epidural analgesia for maintaining mobility after a low-dose combined spinal-epidural. Br J Anaesth.1999:82:233-236.