Research Article - Onkologia i Radioterapia ( 2021) Volume 15, Issue 1
Comparison of toxicities of three weekly and weekly cisplatin in concurrent chemoradiation for head and neck cancers a retrospective cohort study
Ciniraj Raveendran1*, I Yadev2, Krishna Sharan3 and Bejadi Vadhiraja3,42Department of General Surgery, Medical College Thiruvananthapuram, Kerala State, India
3Department of Radiation Oncology, Kasturba Medical College, Manipal, Karnataka State, India
4Department of Radiation Oncology, Manipal Hospital Bangalore, Karnataka State, India
Ciniraj Raveendran, Department of Radiotherapy and Oncology Medical College Thiruvananthapuram Kerala, India, Email: drciniraj@gmail.com
Received: 29-Sep-2020 Accepted: 30-Nov-2020 Published: 12-Dec-2020
Abstract
Background & aims: Concurrent chemoradiation in Head and neck cancer patients offers a significant improvement in local control and overall survival at the expense of added toxicities. In practice, different schedules of Cisplatin are used with concurrent chemoradiation. This retrospective study aims to compare the toxicity profile of head and neck cancer patients treated with concurrent chemoradiation using three weekly cisplatin and weekly cisplatin.
Methods: This is a retrospective cohort study that included patients with Head and Neck Cancers treated with concurrent chemoradiation with either of the two schedules of Cisplatin using 100 mg/m2 every three weeks or 40 mg/m2 every week. Conventional fields were used for radiation treatment. Treatment review charts were used to assess the toxicity of the treatment using RTOG toxicity grading criteria. χ2 test or fishers exact test were used to assess the difference between categorical variables. Continuous variables were compared with the two-sample t-test or Mann Whitney test when assumption could not be satisfied.
Results: A total of 80 patients, of which 56 patients received the three weekly cisplatin while 24 patients had weekly cisplatin with concurrent chemoradiation. Skin toxicity of Grade 3 at treatment completion was 5.4% in the three-weekly chemotherapy group, whereas it was 8.3% in the weekly chemotherapy group, which was statistically significant, (p=0.017). Mucous membrane toxicity was not statistically significant in any of the weeks of treatment. Pharyngeal toxicity reached statistical significance at the 4th week of treatment (p=0.015), but after treatment, no significant difference was seen between groups. Similarly, laryngeal toxicities between the groups were not statistically significant even though higher grade 3 toxicities started appearing from 3rd week. There is a statistically significant difference in terms of toxicities between the two treatment groups in body weight after treatment (p=0.01). When hematological toxicities were considered, hemoglobin toxicity of grade 2 occurred in 16.7% patients in the weekly chemotherapy group, while none at 3rd week which was statistically significant, (p=0.007) while none developed it in the three-weekly group. WBC toxicity showed a significant difference between the groups at the 5th and 6th -week of treatment, p=0.001 and p= 0.04 respectively with higher toxicity and percentages in the weekly chemotherapy group. Platelet toxicity of grade 2 developed in one patient in the weekly chemotherapy, but no statistically significant difference is seen in any of the weeks. The mean radiotherapy delay in days in the three weekly chemotherapy group was 0.36 day (SD 1.67) whereas in the weekly group was 1.33 days (SD 1.67), was not statistically significant. Treatment response was partial in 5.4% in the three weekly chemotherapy group and 4.2% in the weekly chemotherapy group, the difference noted is not statistically significant.
Conclusions: There is heterogenicity in the severity of grade of toxicity within the three-weekly and weekly chemotherapy groups with concurrent chemoradiation. Weekly cisplatin appears more toxic as it is associated with higher grades of toxicities and chemotherapy interruptions. There is no evidence to show that weekly cisplatin is less toxic when compared to three weekly cisplatin in concurrent chemoradiation for head and neck cancers.
Keywords
head and neck cancer, chemo-radiation, toxicities, three weekly chemotherapy, weekly chemotherapy, cisplatin
Introduction
Patients with Head and Neck Squamous Cell Carcinomas (HNSCC) often presents at a very advanced stage. The management of locally advanced HNSCC is challenging with different modalities available for treatment including surgery, radiation, and chemotherapy. It is often encountered with poor outcomes when a single modality was used. Most of the cases are inoperable due to the very advanced stage, age, poor nutrition status, poor oral hygiene, tobacco use, and other comorbidities affecting their performance status [1, 2]. Unrespectable tumours when treated with radiation alone as a primary modality alone are often encountered with inferior outcomes. Chemotherapy when added to radiation treatment results in significant improvement in survival [3]. The benefit of the addition of chemotherapy therapy to radiation is maximum when it is used concomitantly [4]. Various chemotherapy drugs have been used concurrently with radiation to achieve a good clinical response, but acute and late toxicity is the main concern and there exists a significant association between acute toxicity and overall survival and local control of the disease [5, 6]. Drugs like cisplatin, 5 fluorouracil, cetuximab have been used concurrently with radiation, but cisplatin is the most widely used drug. Cisplatin is moderately tolerated and is being administered in varying schedules which includes daily dosing, weekly once, and once in a three-week dose schedule. The doses of each schedule vary with the frequency of administration; the optimal dose schedule is undetermined. The dose of cisplatin at a rate of 100 mg/m2 is the one which is widely used with randomized clinical trials but is associated with significant toxicities and the selection of patients who benefit from this schedule is critical [3,7,8]. World over attempts is being made to reduce the dose of cisplatin to improve treatment compliance, without adverse treatment outcome. The dose of cisplatin at a rate of 40 mg/ m2 every week during radiation treatment is used by several institutions without loss of efficacy with a tolerable toxicity profile, although the long term outcome of this approach needs to be validated in larger randomized clinical trials [9, 10]. The reports on the toxicity profile of the weekly low dose cisplatinbased concurrent chemo-radiation are often heterogeneous and sometimes conflicting, but overall survival and recurrence-free survival are similar [11].
This retrospective study aims to compare the differences in toxicities of patients who received either of the two schedules of cisplatin, with a three-weekly cycle at a dose of 100 mg/ m2 or cisplatin with a weekly cycle at a dose of 40 mg/m2 with concurrent chemo-radiation in the treatment of HNSCC. The primary objective is to compare the toxicity profile, while secondary objectives are to compare the treatment interruptions and response rate.
Materials and Methods
We conducted a retrospective cohort study on HNSCC patients treated with concurrent chemo-radiotherapy at two tertiary care centres in southern India. We conducted this study after obtaining approval from the institutional ethics committee. We included all patients satisfying the inclusion and exclusion criteria. Patients should have received either of the two different schedules of cisplatin chemotherapy i.e. who received threeweekly chemotherapy or weekly chemotherapy Histologic proof of squamous cell carcinoma was mandatory. Non-metastatic disease with stage II, stage III, and stage IV were included in the study. Karnofsky Performance Status of ≥ 60 and age ≥ 20 years and creatinine clearance (CrCl)>60 ml/min before chemotherapy was mandatory. Patients aged more than 70 years, pregnant women, recurrent or metastatic disease, and patients who received prior chemotherapy for any other disease or radiation to the head and neck area were excluded.
All patients underwent staging work up as per institutional protocol which included detailed physical examination, Ear Nose Throat (ENT) examination, Computed Tomography (CT), or Magnetic Resonance Imaging (MRI) of the head and neck. Metastatic workup included chest X-ray in all patients or CT thorax in required cases. Routine blood examination included complete blood count and biochemical tests for renal and hepatic functions. American Joint Committee on Cancer (AJCC) staging was used to stage the disease. The chemotherapy and radiotherapy received were the ones routinely used for patients.
Cisplatin dose was 100 mg/m2 for the three-weekly schedule (D1, D22, D43) and 40 mg/m2 for the weekly schedule. For the three weekly cisplatin chemotherapy vigorous pre-chemotherapy and post-chemotherapy hydration schedule were used along with antiemetic medications and dexamethasone. Along with prechemotherapy hydration, potassium chloride and magnesium sulfate were used intravenously. For diuresis 100 ml of 20%, Mannitol intravenous was given. Cisplatin was diluted in 1 liter of normal saline and infused over 2 hours for the three-weekly schedule, whereas it was 1 hour for the weekly regime. Since the three weekly schedules had a higher dose, stringent postchemotherapy hydration and antiemetic medication were used. Details of chemotherapy administration were obtained from records.
Chemotherapy administration was postponed if any of the haematological or biochemical parameters have nor recovered to the standard chemotherapy administration values. Haemoglobin level >10 gm/dl, absolute neutrophil count >1.5 ×109/L, platelet values >100 × 109/L, and normal renal function was required for chemotherapy administration, all of which was assessed weekly, data of which was obtained from inpatient records.
All patients underwent radiation treatment as per institutional protocols. The radiation fields used are were as of that for the conventional treatment, which included two parallel opposed beams for the face and neck and the third low anterior neck beam to cover the lower neck. The primary disease and high-risk areas have prescribed a dose of 66 Gy in 33 fractions and lower neck to a dose of 50 Gy in 25 fractions, with 2 Gy per fraction, one fraction per day, and 5 days a week treatment. After 44 Gy the field size was reduced to shield the spinal cord.
We reviewed the details of weekly toxicity assessment contained in the weekly review charts of patients for assessing and recording toxicities that occurred during treatment time. RTOG (Radiation Therapy Oncology Group) acute toxicity scoring criteria were used for grading the toxicity of patients during the weeks of treatment. For all patients, weekly monitoring of complete hemogram, biochemical tests including renal and hepatic functions were done. Mucous membrane toxicities, pharyngeal toxicities, laryngeal toxicities, and skin toxicities were the non-hematological toxicities reviewed. Haematological toxicities recorded were Haemoglobin (Hb) levels, White Blood Cell (WBC) counts, and platelet counts. Bodyweight during treatment was also obtained. Grading of toxicity was done using RTOG toxicity criteria ranging from grade 0 to 4 with increasing severity [12]. Radiotherapy delay if any is calculated from the expected day of completion to the actual day of completion. Missed chemotherapy is the actual number of chemotherapy cycles avoided due to unacceptable toxicity or poor tolerance. Response assessment was routinely done for all patients at completion of treatment with RECIST (Response Evaluation Criteria in Solid Tumours) criteria (Figure 1) [13].
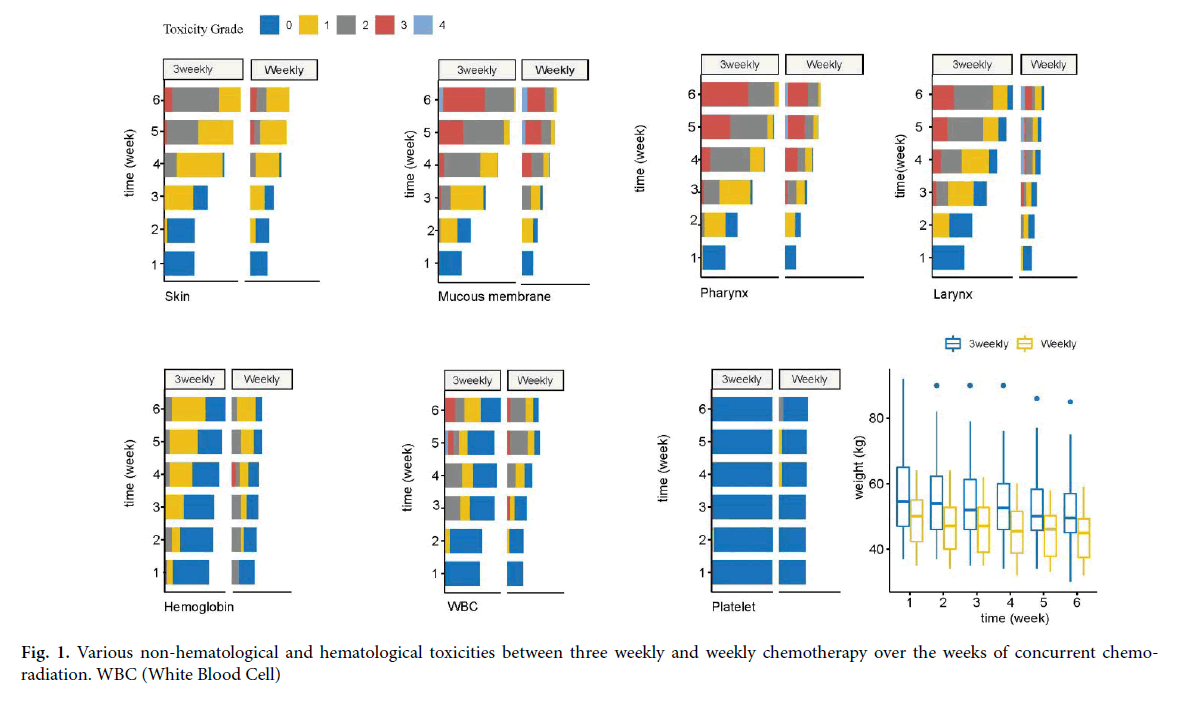
Figure 1: Various non-hematological and hematological toxicities between three weekly and weekly chemotherapy over the weeks of concurrent chemoradiation. WBC (White Blood Cell)
Statistical analysis
Qualitative data were described in terms of proportions and frequencies. Continuous data were described in mean and standard deviation or median and interquartile range. We used the χ2 test or fishers exact test to assess the difference between categorical variables. Continuous variables were compared with the two-sample t-test or Mann Whitney test when assumption could not be satisfied. Statistical significance was set at p<0.05. We used R statistical software for analysing the data [14]. Regression model by using SPSS v22. p<0.05 was deemed significant.
Results
A total of 80 patients received concurrent chemo-radiation for HNSCC with either of the two schedules of cisplatin i.e. three weekly or weekly cisplatin. The median age of patients was 50 years with Interquartile Range (IQR) 45.00, 60.00. Males constitute the majority (77.5%). The commonest site involved by the tumour was the tongue (41.2%) followed by buccal mucosa (13.8%). For histo-pathological type, moderately differentiated and well-differentiated squamous cell carcinoma constituted the majority (45% and 43.8% respectively). The majority of the patients were in stage T3 and N2 (37.5% each) and composite stage IVA and stage IVB (62.5% both included).
The chemotherapy schedule was three-weekly in 56 patients whereas it was a once-weekly regimen in 24 patients. For the three-weekly chemotherapy, the median age was 50 years IQR 45.00,58.5 whereas for the weekly chemotherapy the median age was 49.5 years IQR 40.75,60.00 When the gender was considered males predominated in both groups.
The predominant site of disease was tongue in both groups. Predominant was T2 and T3, T4, and N1 and N2 disease in both groups. When the composite stage was considered stage IV predominated (73.2%) in the three weekly chemotherapy group whereas stage III disease (50%) in the weekly chemotherapy group. The predominant histo-pathological type was moderately differentiated squamous cell carcinoma and well-differentiated squamous cell carcinoma in both groups (Table 1).
Tab.1. Patient characteristics
| Three weekly chemotherapy | Weekly chemotherapy | P | |
|---|---|---|---|
| Number (n) | 56 | 24 | |
| Age Median (IQR) | 50.00 (45.00, 58.50) | 49.50 (40.75, 60.00) | 0.427 |
| Sex=Male (%) | 46 (82.1) | 16 (66.7) | 0.22 |
| SITE (%) | 0.059 | ||
| Buccal mucosa | 7 (12.5) | 4 (16.7) | |
| Glottis | 1 (1.8) | 1 (4.2) | |
| Maxilla | 1 (1.8) | 0 (0.0) | |
| Nasal cavity | 0 (0.0) | 1 (4.2) | |
| Post cricoid | 1 (1.8) | 6 (25.0) | |
| Posterior pharyngeal wall | 2 (3.6) | 2 (8.3) | |
| Pyriform sinus | 3 (5.4) | 1 (4.2) | |
| Retromolar trigone | 4 (7.1) | 1 (4.2) | |
| Soft palate | 2 (3.6) | 0 (0.0) | |
| Supraglottis | 5 (8.9) | 2 (8.3) | |
| Tongue | 28 (50.0) | 5 (20.8) | |
| Tonsil | 2 (3.6) | 1 (4.2) | |
| T Status (%) | 0.162 | ||
| T2 | 12 (21.4) | 10 (41.7) | |
| T3 | 22 (39.3) | 8 (33.3) | |
| T4 | 22 (39.3) | 6 (25.0) | |
| N Status (%) | 0.346 | ||
| N0 | 15 (26.8) | 6 (25.0) | |
| N1 | 16 (28.6) | 11 (45.8) | |
| N2 | 24 (42.9) | 6 (25.0) | |
| N3 | 1 (1.8) | 1 (4.2) | |
| Stage (%) | 0.007 | ||
| II | 5 (8.9) | 3 (12.5) | |
| III | 10 (17.9) | 12 (50.0) | |
| IV | 41 (73.2) | 9 (37.5) | |
| Histopathology (%) | 0.547 | ||
| Moderately differentiated squamous cell carcinoma | 25 (44.6) | 11 (45.8) | |
| Poorly differentiated squamous cell carcinoma | 5 (8.9) | 4 (16.7) | |
| Well-differentiated squamous cell carcinoma | 26 (46.4) | 9 (37.5) |
At the 6th week of treatment grade-3 skin toxicity was seen in 5.4% the three-weekly chemotherapy group compared to 8.3% in the weekly chemotherapy group. When all grades of skin toxicities were considered in the 6th week, the difference between the groups was statistically significant (p=0.017). Mucous membrane toxicity of grade 3 grade 4 toxicity developed in both groups. No statistically significant difference could be observed between the groups in any of the weeks of treatment. As the treatment progressed the pharyngeal toxicity increased in severity to the level that by 6th - week grade 3 toxicity was 50% in both the groups. One patient in the weekly chemotherapy group had grade 4 pharyngeal toxicity. The difference noted between the two groups was statistically significant in the 4th week of treatment (p=0.015). Laryngeal toxicity of grade 3 started appearing in the 3rd week in both groups and reached the maximum by 6th week. One patient in the weekly chemotherapy had grade 4 laryngeal toxicity which occurred in the 4th week and continued till completion of treatment. The differences between the groups are not statistically significant (Table 2).
Tab. 2. Non Hematological toxicities between the two groups over the weeks of treatment
| Acute toxicity | Three weekly chemotherapy | Weekly chemotherapy | p-value |
|---|---|---|---|
| Skin | 0.146 | ||
| WK 2 (%) G1 | 1 (1.8) | 3 (12.5) | |
| WK 3 (%) G1 | 27 (48.2) | 10 (41.7) | 0.769 |
| WK 4 (%) G2 | 7 (12.5) | 2 (8.3) | 0.244 |
| WK 5(%) G2; G3 | 20 (35.7); 1 (1.8) | 3 (12.5); 1 (4.2) | 0.101 |
| WK 6 (%) G2; G3 | 31 (55.4); 3 (5.4) | 5 (20.8); 2 (8.3) | 0.017 |
| Mucous membrane | |||
| WK 2 (%) G2 | 1 (1.8) | 0 (0.0) | 0.497 |
| WK 3 (%) G2; G3 | 8 (14.3); 1 (1.8) | 6 (25.0); 0 (0.0) | 0.159 |
| WK 4 (%) G2; G3 | 30 (53.6); 3 (5.4) | 10 (41.7); 5 (20.8) | 0.075 |
| WK 5 (%) G3; G4 | 15 (26.8); 0 (0.0) | 10 (41.7); 1 (4.2) | 0.107 |
| WK 6 (%) G3; G4 | 26 (46.4); 2 (3.6) | 11 (45.8); 2 (8.3) | 0.606 |
| Pharynx | |||
| WK 2 (%) G2 | 2 (3.6) | 0 (0.0) | 0.633 |
| WK 3 (%) G2; G3 | 13 (23.2); 1 (1.8) | 7 (29.2); 1 (4.2) | 0.14 |
| WK 4 (%) G2; G3 | 32 (57.1); 5 (8.9) | 6 (25.0); 7 (29.2) | 0.015 |
| WK 5 (%) G3; G4 | 17 (30.4); 0 (0.0) | 10 (41.7); 1 (4.2) | 0.205 |
| WK 6 (%) G3; G4 | 28 (50.0); 0 (0.0) | 12 (50.0); 1 (4.2) | 0.576 |
| Larynx | |||
| WK 2 G 2 (%) | 0 (0.0) | 1 (4.2) | 0.305 |
| WK 3 G 3 (%) | 1 (1.8) | 1 (4.2) | 0.513 |
| WK 4 G3; G4 (%) | 3 (5.4); 0 (0.0) | 1 (4.2); 1 (4.2) | 0.069 |
| WK 5 G3; G4 (%) | 6 (10.7); 0 (0.0) | 1 (4.2); 1 (4.2) | 0.257 |
| WK 6 G3; G4 (%) | 9 (16.1); 0 (0.0) | 5 (20.8); 1 (4.2) | 0.066 |
WK: Week; G:Grade of toxicity
Both groups had a downward trend in body weight. The median body weight at the 6th week of completion of concurrent chemo-radiation was statistically significant between the groups (p=0.01). Weight loss of 10% occurred in both groups at completion of treatment (Table 3).
Tab. 3. Bodyweight across treatment groups during the weeks of concurrent chemo-radiation.
| Three weekly chemotherapy | Weekly chemotherapy | P | |
|---|---|---|---|
| WT 1 WK | 54.50 [47.00, 65.00] | 50.00 [42.25, 55.00] | 0.011 |
| WT 2 WK | 54.00 [46.00, 62.25] | 47.00 [40.00, 52.75] | 0.01 |
| WT 3 WK | 52.00 [46.00, 61.25] | 47.00 [39.00, 52.75] | 0.007 |
| WT 4 WK | 52.50 [46.00, 60.00] | 45.50 [38.75, 51.50] | 0.005 |
| WT 5 WK | 50.00 [45.75, 58.25] | 46.00 [37.75, 50.25] | 0.006 |
| WT 6 WK | 49.50 [45.00, 57.00] | 45.00 [37.50, 49.25] | 0.01 |
WT: Body Weight; WK: Week of Treatment
Hemoglobin toxicity of grade 2 occurred in 16.7% in the weekly chemotherapy group and the difference noted between the groups was statistically significant (p=0.007). At 4th week, one patient in the weekly chemotherapy group developed grade 3 haemoglobin toxicity. For all other weeks, the difference between the groups was not statistically significant. WBC toxicity of grade 3 toxicity started appearing early in the 3rd week in one patient in the weekly chemotherapy group. WBC toxicity progressed during the 5th and 6th week of
Treatment. WBC toxicity of grade 4 occurred in one patient in the three weekly chemotherapy groups in the 5th week. The difference noted between the groups is statistically different in the 5th and 6th weeks (p=0.001 and p=0.04 respectively). Platelet toxicity of grade 1 developed in 1 patient in the three weekly chemotherapy group and weekly chemotherapy group. Grade 2 platelet toxicity occurred in 1 patient in the weekly chemotherapy group. The difference between the groups is not statistically significant (Table 4).
Tab. 4. Hematological toxicities between the two groups over the weeks of treatment
| Hematological toxicity | Three weekly chemotherapy | Weekly chemotherapy | P |
|---|---|---|---|
| Hemoglobin | |||
| WK 2 G2 (%) | 3 (5.4) | 4 (16.7) | 0.257 |
| WK 3 G2 (%) | 0 (0.0) | 4 (16.7) | 0.007 |
| WK 4 G2; G3 (%) | 2 (3.6); 0 (0.0) | 2 (8.3); 1 (4.2) | 0.355 |
| WK 5 G2 (%) | 2 (3.6) | 4 (16.7) | 0.104 |
| WK 6 G2 (%) | 3 (5.4) | 2 (8.3) | 0.491 |
| White Blood cell | |||
| WK 2 G2 (%) | 1 (1.8) | 0 (0.0) | 0.8 |
| WK 3 G2; G3 (%) | 8 (14.3); 0 (0.0) | 0 (0.0); 1 (4.2) | 0.106 |
| WK 4 G2 (%) | 9 (16.1) | 4 (16.7) | 0.379 |
| WK 5 G2; G3; G4 (%) | 3 (5.4); 2 (3.6), 1 (1.8) | 9 (37.5); 1 (4.2); 0 (0.0) | 0.001 |
| WK 6 G2; G3 (%) | 5 (8.9); 4 (7.1) | 8 (33.3); 1 (4.2) | 0.044 |
| Platelet | |||
| WK 2 G1 (%) | 1 (1.8) | 0 (0.0) | 1 |
| WK 3 G0 (%) | 56 (100.0) | 24 (100.0) | NA |
| WK 4 G1 (%) | 0 (0.0) | 1 (4.2) | 0.661 |
| WK 5 G1 (%) | 0 (0.0) | 1 (4.2) | 0.661 |
| WK 6 G2 (%) | 0 (0.0) | 1 (4.2) | 0.661 |
WK: Week; G: Grade of toxicity
In the three-weekly chemotherapy group, most patients were able to complete all the planned three cycles of cisplatin chemotherapy (76.8%), while 23.2% of patients missed one cycle of chemotherapy. In the weekly chemotherapy group, only 16.7% of patients were able to complete the planned 6 cycles of chemotherapy. The majority of the patients in the weekly chemotherapy group missed one cycle (20.8%) or two cycles of chemotherapy (38.7%). About 16.7% of patients missed 3 cycles of chemotherapy in the weekly group. Two patients missed 4 cycles of chemotherapy in the weekly chemotherapy group. When the number of missed chemotherapies is considered, the difference between the two groups is statistically significant (p<0.001). The mean radiotherapy delay in days in the three weekly chemotherapy group was 0.36 (SD 1.67) whereas in the weekly group was 1.33 (SD 1.67), was not statistically significant. Treatment response at the end of treatment showed a complete response in most of the patients in both groups. The response was partial in 5.4% in the three weekly chemotherapy group and 4.2% in the weekly chemotherapy group, the difference noted is not statistically significant (Table 5).
Tab. 5. Treatment interruption and response to treatment
| 3-weekly chemotherapy | Weekly chemotherapy |
p-value | |
|---|---|---|---|
| Radiotherapy delay (days) mean (SD) | 0.36 (1.67) | 1.33 (2.81) | 0.057 |
| No of missed chemotherapy (%) | <0.001 | ||
| 0 | 43 (76.8) | 4 (16.7) | |
| 1 | 13 (23.2) | 5 (20.8) | |
| 2 | 0 (0.0) | 9 (37.5) | |
| 3 | 0 (0.0) | 4 (16.7) | |
| 4 | 0 (0.0) | 2 (8.3) | |
| Partial response (%) | 3 (5.4) | 1 (4.2) | 1 |
Discussion
This is a retrospective observational study on patients who received either of the two schedules of chemotherapy with concurrent chemo-radiation i.e. cisplatin at a dose of 100 mg/ m2 administered every three weeks to complete 3 cycles or at a dose of 40 mg/m2 administered every week to complete 6 cycles and compared the two groups in terms of toxicity. Treatmentrelated toxicity leads to treatment interruption, delaying either chemotherapy or radiation, and sometimes both leading to a poor outcome. In this study, most of the patients had grade 1 and grade 2 skin toxicity throughout treatment. Grade 3 toxicity started appearing in the 5th week of treatment, affecting a greater number of patients in the final weeks of treatment. These study results are similar to the study which reported skin toxicity of grade 1 in up to 56% and grade 2 in up to 18% which constituted the majority of the patients and Grade 3 skin reactions in 8-16% patients [15]. Mucous membrane toxicity manifested as oral mucositis is one dreaded toxicity of concurrent chemo-radiation in head and neck cancer and grade 3 toxicities lead to an interruption in treatment. The percentage of grade 3 mucositis occurring varies with the level of oral care they receive during treatment. In this study, grade 3 and grade 4 mucositis started appearing in the 3rd and 5th weeks respectively with an increasing percentage of patients getting affected thereafter. The higher percentage of grade 3 and grade 4 mucositis is comparable to the study which reported a grade 3 oral mucositis in 14%- 34% of their patients [16]. Additionally, in our study grade-4 mucositis was more frequent with weekly chemotherapy, which is expected, because of higher percentages of grade 3 mucositis seen. Pharyngeal toxicity manifested by dysphagia leads to poor oral intake leading to nutritional deficiency, weight loss, and poor outcome [17,18]. In this study, nearly 50% of patients irrespective of the chemotherapy schedule developed grade 3 pharyngeal toxicity in the final week of treatment which is comparable to the reported grade 3 adverse effect in 49% patients by Cooper et al. [19]. Higher percentages of laryngeal toxicity of grade 3 are seen in both the groups of patients, reaching up to 20% of patients in the weekly chemotherapy arm. Even grade 4 laryngeal toxicity occurred in the weekly chemotherapy group. Other studies reported a grade 3 laryngeal toxicity in the range of 10%-17% and majority (80%-90%) had only grade 2 laryngeal toxicity [20, 21]. The higher incidence of laryngeal toxicity in this study may be due to the radiation field used in the treatment without laryngeal shield, which might have contributed a higher dose to the larynx.
In this study, weight loss occurs in patients irrespective of the chemotherapy schedule, the median weight decreased gradually over the weeks of treatment. The median weight decreased by 5 kg in both groups at the end of treatment. The weight loss in this range occurred in other studies also, more than 20% weight loss from the pre-treatment levels lead to treatment interruption [22, 23]. None of the patients in this study had a weight loss of that intensity; still, significant weight loss occurred in patients irrespective of the schedule. This may be due to the lack of nutrition support and calorie monitoring during treatment.
Hemoglobin toxicity manifested as anaemia confined mostly to grade 1 and grade 2 toxicity in this study and only one patient had grade 3 hemoglobin toxicity. A decrease in hemoglobin of this grade is seen in similarly treated group of patients and is comparable to similar studies which reported grade 1 and grade 2 hemoglobin toxicity in 70%-90% patients [21, 24]. The weekly chemotherapy group had higher percentages of grade 2 WBC toxicity manifested as neutropenia at 5th and 6th week of treatment, 37.5%, and 33.3% respectively. This indicates that the weekly schedules tend to have more toxicities nearing completion of treatment. This may be due to frequent administration of cisplatin every week leading to bone marrow suppression and insufficient time to recover. This is in contrast with other studies which reported grade 2 WBC toxicity in 40%-80% patients and grade 3 WBC toxicity in 5%-20% patients in the weekly chemotherapy schedule, which may be due to the lower dose of cisplatin received in the three weekly regimen, unplanned chemotherapy omissions or lower dose of cisplatin used in the weekly regimens leading to such toxicity profile in those studies [15, 20, 21]. Platelet toxicity manifested as thrombocytopenia occurred in a lesser number of patients in both groups, restricting itself to grade1 and grade 2. The weekly chemotherapy group had more grade 1 and grade 2 platelet toxicity 4.2% each which were self-limiting. Other studies also reported that platelet toxicity of grade ≤ 2 in 40%-90% of patients [21, 24].
In this study majority (76.8%) in the three weekly were able to complete the planned 3 cycles of chemotherapy along with radiation, which reported an acceptance rate of 65% with three weekly cisplatin dosing schedules [15, 25]. Patients who received weekly chemotherapy should have had a total of 6 cycles of chemotherapy during the treatment. But in the weekly chemotherapy group, only 16.7% were able to complete all the 6 cycles of chemotherapy and the majority (37.5%) missed two cycles of chemotherapy. The compliance to weekly chemotherapy was good at the beginning of treatment, worsened as the treatment progressed leading to omissions in chemotherapy. Such omissions are not uncommon in the weekly chemotherapy regimens, even then the majority (75%) of the patients were able to complete at least 4 cycles of chemotherapy. Other studies reported a similar compliance rate after the 4th cycle of chemotherapy, due to toxicities associated with treatment which lead to the omission of chemotherapy cycles [15, 21]. There are minor variations in the compliance rate reported in concurrent chemo-radiation studies that used weekly cisplatin which may be due to the dose of cisplatin used in those studies varying from 30 mg/m2 to 50 mg/m2 [26-28].
When the delay in completion of radiation treatment was compared between the three weekly chemotherapy group and weekly chemotherapy group, no significant difference could be found between the groups. Other studies also reported mean radiation interruption is 4.1 days to 5 days without prolonging the duration of the entire treatment in the majority of patients [16, 29]. This may be because most patients are continued on their radiation treatment if they could tolerate it by omitting one or two cycles of chemotherapy to prevent gaps in treatment, which it occurs will lead to poor response. The response at the end of treatment did not differ significantly with most of the patients achieving a complete response leaving partial response only in 5.4 % in the three weekly chemotherapy groups and 4.2% in the weekly chemotherapy group. The results of the response rate of this study are comparable to other studies that reported a partial response in 8%-14% of patients [21, 30, 31].
Reporting of toxicities in head and neck cancers is not seen in all studies on chemo-radiation. Not many studies are available on weekly chemotherapy with radiation which reports toxicities in head and neck cancers. Our study concentrates on the toxicity aspect of the treatment, even more strength with the assessment of toxicities with the available data on all weeks of treatment. There is a potential for selection bias in our study, the selection of chemotherapy regimen was the physician’s choice, and any effect on its outcome is unknown. There are differences in the baseline characteristics of the patients; its effect on toxicity outcome is unknown. Being a retrospective study with less sample size is a weakness of the study. Data to assess the long-term toxicities and the outcome was not available. A randomized control trial with enough power could answer these questions correctly.
Conclusion
Concurrent chemo-radiation with cisplatin in head and neck squamous cell carcinoma is associated with various acute toxicities. There is heterogenicity in the severity of the grade of toxicity within the groups as the treatment progressed. With concurrent chemo-radiation, the weekly chemotherapy appears more toxic as higher grades of toxicities and chemotherapy interruptions occur more frequently. Radiation delays are minimal and treatment outcome is similar to both chemotherapy schedules. However, there is no evidence to say that weekly cisplatin chemotherapy with concurrent chemo-radiation is less toxic than three weekly cisplatin.
Financial Support and Sponsorship
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgement
The authors declare that there are no conflicts of interest.
References
- Somani N, Goyal S, Pasricha R, Khuteta N, Agarwal P, et al. Sequential therapy (triple drug-based induction chemotherapy followed by concurrent Chemo-radiotherapy) in locally advanced inoperable head and neck cancer patients - Single institute experience. Indian J Med Paediatr Oncol. 2011;32:86-91.
- Suresh AV, Varma PP, Sinha S, Deepika S, Raman R, et al. Risk-scoring system for predicting mucositis in patients of head and neck cancer receiving concurrent Chemo-radiotherapy [rssm-hn]. J Cancer Res Ther. 2010;6:448-451.
- Adelstein DJ, Li Y, Adams GL, Wagner H, Jr., Kish JA, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent Chemo-radiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21:92-98.
- Pignon JP, le Maitre A, Maillard E, Bourhis J, Group M-NC. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4-14.
- 5. Bhide SA, Ahmed M, Barbachano Y, Newbold K, Harrington KJ, et al. Sequential induction chemotherapy followed by radical chemo-radiation in the treatment of locoregionally advanced head-and-neck cancer. Br J Cancer. 2008;99:57-62.
- 6. Wolff HA, Daldrup B, Jung K, Overbeck T, Hennies S, et al. High-grade acute organ toxicity as positive prognostic factor in adjuvant radiation and chemotherapy for locally advanced head and neck cancer. Radiology. 2011;258:864-871.
- Lee JH, Song JH, Lee SN, Kang JH, Kim MS, et al. Adjuvant Postoperative Radiotherapy with or without Chemotherapy for Locally Advanced Squamous Cell Carcinoma of the Head and Neck: The Importance of Patient Selection for the Postoperative Chemo-radiotherapy. Cancer Res Treat. 2013;45:31-39.
- Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091-2098.
- Ghosh S, Rao PB, Kumar PR, Manam S. Weekly Cisplatin-Based Concurrent Chemo-radiotherapy for Treatment of Locally Advanced Head and Neck Cancer: a Single Institution Study. Asian Pac J Cancer Prev. 2015;16:7309-7313.
- Watkins JM, Zauls AJ, Wahlquist AH, Shirai K, Garrett-Mayer E, et al. Low-dose weekly platinum-based chemo-radiation for advanced head and neck cancer. Laryngoscope. 2010;120:236-242.
- Guan J, Zhang Y, Li Q, Zhang Y, Li L, et al. A meta-analysis of weekly cisplatin versus three weekly cisplatin chemotherapy plus concurrent radiotherapy (CRT) for advanced head and neck cancer (HNC). Oncotarget. 2016;7:70185-70193.
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995; 31:1341-1346.
- Schwartz LH, Litiere S, de Vries E, Ford R, Gwyther S, et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur J Cancer. 2016;62:132-137.
- Team RC: R: A Language and Environment for Statistical Computing.R Foundation for Statistical Computing, Vienna, Austria; 2020.
- Geeta SN, Padmanabhan TK, Samuel J, Pavithran K, Iyer S, et al. Comparison of acute toxicities of two chemotherapy schedules for head and neck cancers. J Cancer Res Ther. 2006;2:100-104.
- Suhag V, Sunita BS, Vats P, Chakravarty N, Pandya T, et al. Tolerance of Chemo-radiation in Advanced Head and Neck Cancers: Comparison Between Inpatients and Outpatients. Indian J Otolaryngol Head Neck Surg. 2019;71:192-198.
- Yanni A, Dequanter D, Lechien JR, Loeb I, Rodriguez A, et al. Malnutrition in head and neck cancer patients: Impacts and indications of a prophylactic percutaneous endoscopic gastrostomy. Eur Ann Otorhinolaryngol Head Neck Dis. 2019;136:S27-S33.
- Brown TE, Banks MD, Hughes BGM, Lin CY, Kenny LM, et al. Randomised controlled trial of early prophylactic feeding vs standard care in patients with head and neck cancer. Br J Cancer. 2017;117:15-24.
- Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937-1944.
- Mashhour K, Hashem W. Cisplatin Weekly Versus Every 3 Weeks Concurrently with Radiotherapy in the Treatment of Locally Advanced Head and Neck Squamous Cell Carcinomas: What Is the Best Dosing and Schedule? Asian Pac J Cancer Prev. 2020;21:799-807.
- Nanda R, Katke A, Suneetha N, Thejaswini B, Pasha T, et al. A prospective randomized study comparing concurrent chemo-radiation with weekly and 3 weekly cisplatin in locally advanced oropharyngeal carcinoma. South Asian Journal of Cancer. 2019;8:178-182.
- Capuano G, Grosso A, Gentile PC, Battista M, Bianciardi F, et al. Influence of weight loss on outcomes in patients with head and neck cancer undergoing concomitant Chemo-radiotherapy. Head and Neck. 2008;30:503-508.
- van den Berg MGA, Rasmussen-Conrad EL, Gwasara GM, Krabbe PFM, Naber AHJ, et al. A prospective study on weight loss and energy intake in patients with head and neck cancer, during diagnosis, treatment and revalidation. Clinical Nutrition (Edinburgh, Scotland). 2006; 25:765-772.
- Mackiewicz J, Rybarczyk-Kasiuchnicz A, Łasińska I, Mazur-Roszak M, Świniuch D, et al. The comparison of acute toxicity in 2 treatment courses: Three-weekly and weekly cisplatin treatment administered with radiotherapy in patients with head and neck squamous cell carcinoma. Medicine (Baltimore). 2017;96:e9151.
- Ho KF, Swindell R, Brammer CV. Dose intensity comparison between weekly and 3-weekly Cisplatin delivered concurrently with radical radiotherapy for head and neck cancer: A retrospective comparison from New Cross Hospital, Wolverhampton, UK. Acta Oncologica. 2008; 47:1513-1518.
- Szturz P, Wouters K, Kiyota N, Tahara M, Prabhash K, et al. Weekly Low-Dose Versus Three-Weekly High-Dose Cisplatin for Concurrent Chemo-radiation in Locoregionally Advanced Non-Nasopharyngeal Head and Neck Cancer: A Systematic Review and Meta-Analysis of Aggregate Data. Oncologist. 2017;22:1056-1066.
- Hayashi Y, Minamiyama S, Ohya T, Iida M, Iwai T, et al. Daily Cisplatin and Weekly Docetaxel versus Weekly Cisplatin Intra-Arterial Chemo-radiotherapy for Late T2-3 Tongue Cancer: A Pilot and Feasibility Trial. Medicina (Kaunas). 2018;54.
- Ho KF, Swindell R, Brammer CV. Dose intensity comparison between weekly and 3-weekly Cisplatin delivered concurrently with radical radiotherapy for head and neck cancer: a retrospective comparison from New Cross Hospital, Wolverhampton, UK. Acta Oncol. 2008; 47:1513-1518.
- Yarn C, Wakefield DV, Spencer S, Martin MY, Pisu M, et al. Insurance status and head and neck radiotherapy interruption disparities in the Mid-Southern United States. Head Neck. 2020;42:2013-2020.
- Lee SY, Choi YS, Song IC, Park SG, Keam B, et al. Comparison of standard-dose 3-weekly cisplatin and low-dose weekly cisplatin for concurrent chemo-radiation of patients with locally advanced head and neck squamous cell cancer: A multicenter retrospective analysis. Medicine (Baltimore). 2018;97:e10778.
- Glaser MG, Leslie MD, O'Reilly SM, Cheesman AD, Newlands ES. Weekly cisplatinum concomitant with radical radiotherapy in the treatment of advanced head and neck cancer. Clin Oncol (R Coll Radiol). 1993; 5:286-289.

