Research - Onkologia i Radioterapia ( 2021) Volume 15, Issue 5
Critical concepts in craniofacial microsurgical reconstruction
Hamid Reza Fathi1, Javad Rahmati1, Arjang Ghahremani1, Jahid Mammadov2 and Mahammad Davudov2*2Department of Oral and Maxillofacial Surgery, Azerbaijan Medical University, Baku, Azerbaijan
Mahammad Davudov, Department of Oral and Maxillofacial Surgery, Azerbaijan Medical University, Baku, Azerbaijan, Email: mahammad_davud@mail.ru
Received: 22-Apr-2021 Accepted: 15-May-2021 Published: 25-May-2021
Abstract
Over the last several decades, there have been numerous advances in the fields of aesthetic, craniofacial, and microsurgery. According to Fisher et al. aesthetic units are no longer "skin deep" but are recognized as being composed of both soft and hard tissue. Indeed, hard tissue must complement the soft tissue to recreate the unit. In addition, revisionary procedures have become necessary to achieve the desired result. We assembled a two-centre, retrospective cohort review of patients who underwent free-tissue transfer of craniofacial defects at the Cancer Institute (Tehran) and the Central Hospital (Baku) from 2009 to 2013. Patients were categorized by anatomic location, complications recorded, and illustrative cases selected. A total of 124 patients with craniofacial defects were identified: 39 female and 85 male patients, with a mean age of 57 years. Etiologies included cancer (95.2%), trauma (0.8%), congenital defects (1.6 %), and benign tumour (2.4%). Free-tissue transfers included 38 fibula, 6 anterolateral thigh, 19 latissimus dorsi musculocutaneous flap, 12 latissimus dorsi muscle flap, 12 osteocutaneous radial, 16 fasciocutaneous, 14 rectus abdominis musculocutaneous, 6 rectus abdominis muscle, one vastus lateralis flaps. The success rate was 96.7% and complication rate was 11.2%. Secondary procedures included fat injection, tissue resuspension, and cutaneous flap excision followed by full-thickness skin grafting or tissue rearrangement. Here, we integrate the critical concepts and provide a patient series illustrating their success.
arion-krmiva kupispredas rottoconsultants hattrennet insurancemarketingpros abcoelectricli paddriver bobcoironrailings cliniquemtarhemodialyse gtech settechny groupe-saturne caffeitaliany rcollision husoghytteplan okba-medicaments mazex atyourserviceoil hetgroenewerk msgfeather rolltech ismllw lorlin conceriacaponigiuseppe chouikha-big dona-hotel ibrax fullthrottleeventplanning theblindspotli mustakynnys curdent auprintemps koulouritispolis floorbufferbrush minicoindustries thespongecompany localvisits fixcars baofoto poi elvisnewman palestragymtonic cavalierifuel unityrubberproductsllc menuiseriemorlighem nugris skillslab mecanica vksim brcanvas handfordoil lemi-yhdistys schoolbuspartsnow thebestofchampaign testingmechanics comm-unique goodnewsbooks generaldecor campye defence-institute fairwaymanorllc whpdc regionalsigns babuin islandeastdentalgroup sirajeslov adda thebestofcleveland integraff sealfiberglass nutechsys tcilandscaping digitaltechsquad tomsvetteshop funda-mantels rahaanopeasti thebowmanfirm marshallcoffeeny mrbrushes sicop-pentacol myguyappliance ldclean ephesusmedcuisine alphafastenersusa unitysurfacing allseasons-mechanical easyketodietsuccess liusaari strongarmcleaningny josg sterlingcutter unadesinfection huizebuitenhuis thebestofraleigh drcentralbaking catsol traditionaltrainsandhobbies goelectricnj casaconcreteinc thebestroofingcompanies autoproautomotiveservice general-machine djsensationalsounds fevaagbaatforening straightlinecustomconstruction impianti-antizanzare thebestoffortworth rosanneebner edandson americanchoirgown violiner jeromeandleighcrutch promisinguk iasautomotive scrittori plasticsrecycling giuseppedainelli jerryspride prontointerventoelettricista24h fimad gdezinewraps kimmokakko tracony fiavet bwnivelles alsalamzorg septimus pdcfundraising hoteldellaspina francobianchi cleansweepcremations errachid thebestofeugene grossoregistratori brotherspastries bellmoreglassandmirror plasmapreen microdecisionsystems eternoholdings alessiocostruzioni hotelpisa excelcourtreporters gmstow tatsrl chateaulamercatering bargaouirideau shcarwash shipritebags allportstrucking juventuspizza red-agri licorneargent thebestofphiladelphia lewisy greenrecuperi thebestofindianapolis fosenlagetsangkor coastweldingsupply nyfixcars alshubcaps hanssenspronkfamilierecht scottsafe barbatonursery jelconst jerryshulmanproduce orchardrealty minervasbandb dialindustries phytosif icredit thebestoftallahassee malermester-blakstad morvayautosiskola tektronicsinc cance-tu-asbl bicotec martinsqualitytruckbody paprikalongbridge roger-jensen rockypointbarbershop rmkdistributors tommiriiulid atlaschemicalllc gopaverinstaller onyxchb thewindowmill khalfallah-pneus ciaociao lexilogistics tipografiaelleemme thebestofwichita cdvdpro roll-n-roaster repelrestoration kcfapi ivar-moe bacosport byggkonsult predicate rohanengineeringpc footpharmacydirect solidbox piovesan visserijverduurzaamt toscanibus sixgsroofing tecnocostruzionizella outsourcemarketingpros pilotexamsdgca carraihome royalbakersdist guardiedicitta bestbaby-tn littlechicken werks1inc hodsonoilco surfacingsystems lckcabinetry pbtools4u set-mfg serristoricountry goldenmoonusa fourcmanagement lioutdoorliving nova-euro-fashion lavecchiacascina pomaraf novamaille dkstechnoholdings liisasauso sahel-tunisie inspired-tech aldamartini monarchengraving craldipendentiuslprato centuryhardware scholengroep ourtowncarwashandquicklube mayablog geometraparisi ash-grove tendertoo smithoilcompany westfrieslanddakbedekkingen lamaisonbeb blog ebiketime laurenty puurklant vincentwielders chams nassausuffolkirrigation bbdps aerocbt fursbysuperior unityrubbercompany horizonconceptinc immstema vhujon nativelandsmokeshop kaabia-orthodontie locali brooklynterminalmarketonline transportopplaering neonmazesl kuturanta apsbox federalnetworks liontrading power-tran degryse-chauffage uniquemasonrycorp proramps catchasilverstar projectbinder unityllc italyvacationpackages rrappliances norwestac topspintennisli boatfindertransport sblcollege imperialvendinginc nanea igiardinidibeatrice pslniemela holycowindian sisekosmosejaam acquadirete obertaberhof thebestofdallas betterheader mrpickleinc medspareparts thebestofsacramento toyotavandergeest alliance-consulting scbox sshoreendo thebestofgreenbay fiducia-partner komunikujeme lapetitemaisonenfrance chesterplastic wisesystems terveysverkko oportal cjflagandson gold-estate spiriolaw salvatorechiarelli autoskola trendcreditcorp scooterkingalmere sisternibedita thebestofminneapolis cerealism transnationalusa homedelano francobenvenutiartista kulsaasvelforening florencevillavioletta gettingerfeathers touchofclasscoll tomkovci
Keywords
cancer, craniofacial, microsurgery, reconstruction
Introduction
The head and neck area is a source of varied and challenging tumours. In the past, these were unrespectable or not reconstructable, or both. With the help of sophisticated imaging, better surgical approaches, the operating microscope, micro vascular composite tissue transplantation, interspecialty cooperation (e.g., neurosurgery, neuro-otology, radiation oncology, and medical oncology), and most of all experience, what seemed impossible is now possible. Plastic surgeons have become head and neck and skull base surgeons [1-6]. Over the last several decades, there have been numerous advances in the fields of aesthetic, craniofacial, and microsurgery. According to Fisher et al. aesthetic units are no longer "skin deep" but are recognized as being composed of both soft and hard tissue. Indeed, hard tissue must complement the soft tissue to recreate the unit. In addition, revisionary procedures have become necessary to achieve the desired result.
Materials and Methods
We assembled a two-center, retrospective cohort review of patients who underwent free-tissue transfer of craniofacial defects at the Cancer Institute (Tehran) and the Central Hospital (Baku) from 2009 to 2013. Patients were categorized by anatomic location, complications recorded, and illustrative cases selected. A total of 124 patients with craniofacial defects were identified: 39 female and 85 male patients, with a mean age of 57 years.
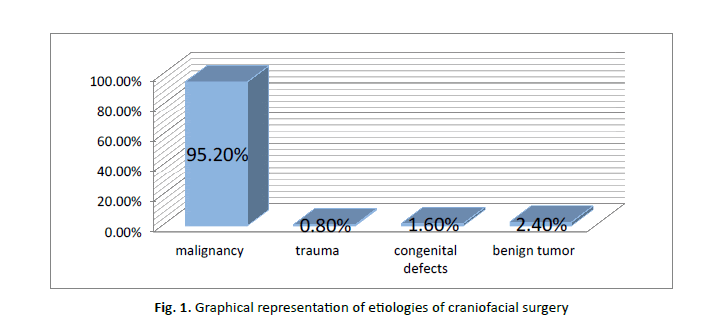
Etiologies included: cancer (95.2%), trauma (0.8%), congenital defects (1.6%), and benign tumour (2.4%). (Table 1) (Figure 1).
Figure 1: Graphical representation of etiologies of craniofacial surgery
Tab. 1. Etiologies of craniofacial surgery
| Etiology | % |
|---|---|
| Malignancy | 95.2% |
| Trauma | 0.8% |
| Congenital defects | 1.6% |
| Benign tumor | 2.4% |
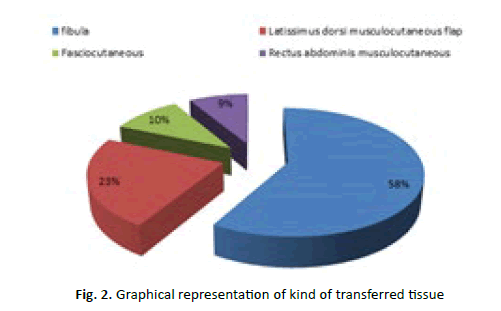
Free-tissue transfers that we used included: 38 fibula, 6 anterolateral thigh, 19 latissimus dorsi musculocutaneous flap, 12 latissimus dorsi muscle flap, 12 osteocutaneous radial, 16 fasciocutaneous radial, 14 rectus abdominis musculocutaneous, 6 rectus abdominis muscle, one vastus lateralis flaps (Table 2) (Figure 2).
Figure 2: Graphical representation of kind of transferred tissue
Tab. 2. Kind of transferred tissue
| Transferred Tissue | Number of procedure |
|---|---|
| Fibula | 38 |
| Anterolateral thigh | 6 |
| Latissimus dorsi musculocutaneous flap | 19 |
| Latissimus dorsi muscle flap | 12 |
| Osteocutaneous radial | 12 |
| Fasciocutaneous radial | 16 |
| Rectus abdominis musculocutaneous | 14 |
| Rectus abdominis muscle | 6 |
| Vastus lateralis flap | 1 |
Graphical representation of kind of transferred tissue
Results
The success rate was 96.7% and complication rate was 11.2%. Secondary procedures included fat injection, tissue resuspension, and cutaneous flap excision followed by full-thickness skin grafting or tissue rearrangement.
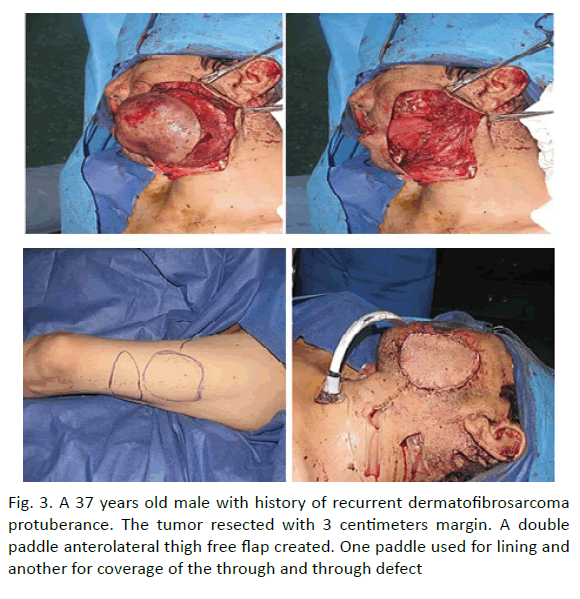
Here, we integrate the critical concepts and provide a patient series illustrating their success. In 6 patients was done reconstruction with anterolateral thigh flap. In 1 patient a double paddle anterolateral thigh free flap created. The tumour resected with 3 centimetres margin. One paddle used for lining and another for coverage of the through and through defect (Figure 3).
Figure 3: A 37 years old male with history of recurrent dermatofibrosarcoma protuberance. The tumor resected with 3 centimeters margin. A double paddle anterolateral thigh free flap created. One paddle used for lining and another for coverage of the through and through defect
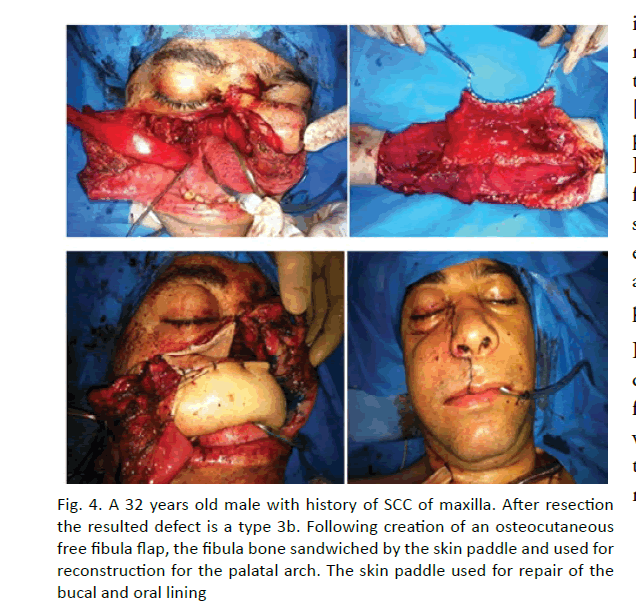
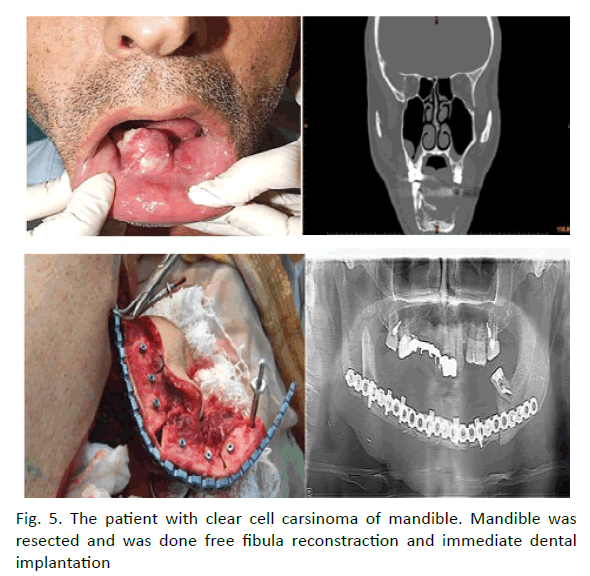
In 38 patients maxillofacial bone defects were reconstructed with fibula free flap. The one of the patients the defect after maxilloectomy a type 3b was reconstructed with osteocutaneous free fibula flap (Figure 4). Following creation of an osteocutaneous free fibula flap, the fibula bone sandwiched by the skin paddle and used for reconstruction for the palatal arch. The skin paddle used for repair of the bucal and oral lining. Routine reconstruction of subtotal defects of the mandible and orthopaedic rehabilitation supported by dental implants in the mean lasting a year. Single stage surgical treatment with immediate orthopaedic rehabilitation was done by help of preoperative virtual computer simulation in two patients. (Figure 5) 3D investigation of pathological and donor sites, virtual simulation of tumour resection, positioning of the dental implants into fibula, virtual flap bending and transfer, virtual bending of fixing reconstruction plates, and fabrication of navigation templates and bridge prosthesis supported by dental implants were done on Pre-op stage. The surgery included tumour resection, insertion of dental implants into fibula and elevation of fibula osteocutaneuose free flap, rigid fixation within recipient site and immediate loading by bridge orthopaedic device. On 10- month follow-up functional and esthetic results were asses as reasonable. Radiology showed dental implants to be integrated and positioned appropriately. We found that successful rehabilitation of the patients with extensive defects of the jaws could achieve by ablative tumour resection, dental implants insertion prior to flap elevation guided by navigation templates, further osteotomy, modelling of the flap according navigation template, flap transfer and rigid fixation within recipient site by prebended plates, with application of prefabricated prosthesis.
Figure 4: A 32 years old male with history of SCC of maxilla. After resection the resulted defect is a type 3b. Following creation of an osteocutaneous free fibula flap, the fibula bone sandwiched by the skin paddle and used for reconstruction for the palatal arch. The skin paddle used for repair of the bucal and oral lining
Figure 5: The patient with clear cell carsinoma of mandible. Mandible was resected and was done free fibula reconstraction and immediate dental implantation
Discussion
Reconstructive maxillofacial surgery refers to the wide range of procedures designed to rebuild or enhance soft or hard tissue structures of the maxillofacial region. Reconstructions of jaw and mouth defects represent a challenge to the surgeon [1-5] and are most commonly indicated in patients with oral Squamous Cell Carcinoma (SCC). They are also used in cases of benign tumours, trauma, osteoradionecrosis, and infection, chronic non-union of bone, clefts, congenital deformities and old age [5-7]. The development of antibiotics, improved diagnostic imaging and anaesthesia have heralded a new era of success in maxillofacial reconstruction [1,2,4,6]. In the past twenty years, the development of bone technology [8-12], osseointegration [13-17] and microsurgery [7,18,19] and improved dental prosthodontics have revolutionised maxillofacial reconstruction. Following surgery, early wound closure and the restoration of form, cosmetics and function are the goals of reconstructive surgery [1,6]. This article seeks to review the modern methods employed in the reconstruction and rehabilitation of the form and function of the jaws and mouth such as free tissue transfer, prosthodontics and dental implants.
In 1970s the unique method for reconstruction of craniofacial defects was included fill the entire hole with soft tissue, and failure rate at this method was nearly 40%. At the present time we can achieve to best results in craniofacial surgery by attention to critical concepts in aesthetic craniofacial microsurgical reconstruction. These concepts are:
• Knowledge of aesthetic units of face
• Defect boundaries
• Tissue requirements
• Bone and soft tissue support
• Soft tissue volume
• Timing
• Secondary revision
In most of the approaches using osteotomies that have been discussed, it is not unusual to perform the bone cuts and the bone shifts that are convenient for the particular exposure required. For this, the term exposure by facial disassembly can be conveniently employed. It is this concept, freedom from rigid boundaries and anatomic terms that has allowed the development of newer and safer methods of exposure. This adaptability has helped establish an "as required" approach, much to the patient's benefit. New frontiers have been opened and crossed. Challenges remain, but as technology improve in terms of sophisticated guidance systems, more precise and effective radiation treatment, and new chemotherapeutic agents together with earlier diagnosis, the outlook for previously incurable conditions continues to improve [20].
Maxillofacial reconstruction is of prime importance in the management of orofacial defects caused by disorders such as neoplastic disease. The modern techniques for reconstruction are discussed below. Vascularised Free Tissue Transfer (VFTT), also known as free flap transfer, is now considered the gold standard for maxillofacial reconstruction [4,6]. It involves the harvesting and detachment of tissue with its blood and nerve supply and transferring it to repair a defect, where its blood and nerve supply are re-established by re-anastomosis to suitable recipient site vessels [6]. Success rates are estimated at between 90% and 94% [20,21]. VFTT is advantageous over non-vascularised transfer, as postoperative radiation affects the vascularized flap less severely compared to the non-vascularised flap due to the transferred blood supply. A number of different donor sites are used for VFTT, the selection of which depends on the recipient site and type of tissue being replaced [11, 12]. The future for maxillofacial reconstruction is bright as a wide range of techniques are being developed to improve upon the advances of the past few decades [6,7].
Conclusion
Orofacial defects can have detrimental functional and psychological effects on the patient. However, in the modern maxillofacial world, the surgeon has a wealth of techniques to draw upon to manage such defects. The management involves either surgical reconstruction or prosthetic rehabilitation or a combination of both. Microsurgery, osseointegration and bone technology have become the keystones in orofacial reconstruction and major advances in recent years have resulted in more treatment modalities and increased success.
References
- Schrag C, Chang YM, Tsai CY, Wei FC. Complete rehabilitation of the mandible following segmental resection. J Surg Oncol. 2006;94:538-545.
- Mukerji R, Mukerji G, McGurk M. Mandibular fractures: historical perspective. Br J Oral Maxillofac Surg. 2006;44:222-228.
- Grusovin MG, Coulthard P, Worthington HV. The efficacy of various bone augmentation procedures for dental implants: a cochrane systematic review of randomized controlled clinical trials. Cochrane Database Syst Rev. 2006;21:696-710.
- Mehta RP, Deschler DG. Mandibular reconstruction in 2004: an analysis of different techniques. Curr Opin Otolaryngol Head Neck Surg. 2004;12:288-293.
- Brown JS, Rogers SN, McNally DN, Boyle M. A modified classification for the maxillectomy defect. Head Neck. 2000;22:17-26.
- Mitchell D. An introduction to oral and maxillofacial surgery. Oxford University Press. 2005:380.
- Urken ML, Weinburg H, Vickery C, Buchbinder D, Lawson W, et al. Oromandibular reconstruction using microvascular composite free flaps, report of 71 cases and a new classification scheme for bony, soft-tissue, and neurologic defects. Arch Otolaryngol Head Neck Surg. 1991:117:733-744.
- Galán GS, Peñarrocha DM, Balaguer MJ, Marti BE. Rehabilitation of severely resorbed maxillae with zygomatic implants: an update. Med Oral Patol Oral Cir Bucal. 2007;12:216-220.
- Ylikontiola L, Sundqvuist K, Sandor GK, Tormala P, Ashammakhi N. Self-reinforced bioresorbable poly-L/DL-lactide 70/30 miniplates and miniscrews are reliable for fixation of anterior mandibular fractures: a pilot study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:312-317.
- Springer IN, Niehoff P, Açil Y, Marget M, Lange A, et al. BMP-2 and bFGF in an irradiated bone model. J Craniomaxillofac Surg. 2008;36;210-217.
- Abukawa H, Papadaki M, Abulikemu M, Leaf J, Vacanti JP, et al. The engineering of craniofacial tissues in the laboratory: a review of biomaterials for scaffolds and implant coatings. Dent Clin North Am. 2006;50:205-216.
- Wan DC, Nacamuli RP, Longaker MT. Craniofacial bone tissue engineering. Dent Clin North Am. 2006;50:175-190.
- Frodel JL, Funk GF, Capper DT, Fridrich KL, Blumer JR, et al. Osseointegrated implants: a comparative study of bone thickness in four vascularised bone flaps. Plast Reconstr Surg. 1993;92:449-458.
- VanSteenberghe D, Lekholm U, Bolender C, Folmer T, Henry P, et al. Applicability of osseointegrated implants in the rehabilitation of partial edentulism: a prospective multicentred study on 558 fixtures. Int J Oral Maxilofac Implants. 1990;5:272-281.
- Paquette DW, Brodala N, Williams RC. Risk factors for endosseous dental implant failure. Dent Clin North Am. 2006;50:361-374.
- Scher E, Holmes S. Simplified transfer of intra oral bone grafts in ridge augmentation procedures. Implant Dent. 2003;12:113-115.
- Leung AC, Cheung KL. Dental implants in reconstructed jaws: patients’ evaluation of functional and quality of life outcomes. J Oral Maxillofac Implants. 2003;18:127-134.
- Hidalgo DA, Pusic AL. Free flap mandibular reconstruction: a 10 year follow up study. Plast Reconstr Surg. 2002;110:438-449.
- Genden E, Haughey BH. Mandibular reconstruction by vascularised free tissue transfer. Am J Otolaryngol. 1996;17:219-227.
- Burkey BB, Coleman JR. Current concepts in oromandibular reconstruction. Otolaryngol Clin North Am. 1997;20:607-630.