Research Article - Onkologia i Radioterapia ( 2021) Volume 15, Issue 2
Effect of Adoptive T cell Therapy on VEGF Signaling Pathway in DMH-Induced Colon Cancer in Balb/c Mice (Lebanon)
Bou Hamdan M, Massaad M, Usta J and Borjac J*2Department of Experimental Pathology, American University of Beirut, Lebanon
3Department of Biochemistry, American University of Beirut, Lebanon
Borjac J, Department of Biological Sciences, Beirut Arab University, Debbieh, Lebanon, Email: j.borjac@bau.edu.lb
Received: 21-Dec-2020 Accepted: 04-Jan-2021 Published: 11-Jan-2021
Abstract
Colon cancer is the fourth most leading causes of cancer-related deaths worldwide accounting for nearly 7-10% of all cancers. Adoptive T Cell Therapy (ACT), a type of immunotherapy, was first developed using Tumor-Infiltrating Lymphocytes (TILs) whereby TILs from the cancer patients are isolated, expanded in vitro using a high concentration of Interleukin-2 (IL-2), and then injected back into the same patients. It was shown that chemotherapy-induced lymphodepletion prior to ACT activates the persistence and anticancer effectiveness of the injected cells. In this study, we aimed to determine the effect of Adoptive T cell therapy (CD8 T cell therapy) alone or in adjunct to Sorafenib on the Vascular Endothelial Growth Factor (VEGF) signaling pathway. CRC was induced in Balb/c mice using dimethylhydrazine injections once per week for 12 consecutive weeks. The mice were treated with either sorafenib, Adoptive T cell therapy or both. Colons were used for histological and molecular analysis. Gene expression was performed using RT-PCR. Sorafenib and/ or Adoptive T cell therapy aided in restoring the normal histology and structure of colon cancer tissue. Sorafenib and/or CD8 T cell treatment led to a decrease in the expression levels of VEGF-A, VEGFR-1, VEGFR-2, BRAF, mTOR, PI3K, KRAS and AKT as compared to untreated control Balb/c mice group. In conclusion, our findings may open up future work on the effect CD8 T cell therapy in colon cancer and on producing new anti-colon cancer therapeutic agents targeting these pathways. This study may be utilized as a base for immunotherapeutic research in colon cancer.
Keywords
Colon cancer, Adoptive immunotherapy, Chemotherapy, VEGF/ VEGFR
Introduction
Colon cancer is one of the most leading causes of death worldwide [1]. It is considered the fourth most leading causes of cancer-related deaths worldwide [1,2], second most common cancer in females, third in males, and the third worldwide [3] accounting for nearly 7-10% of all cancers [4, 5]. In 2014, colon cancer was reported to be the third leading cause of cancer-related deaths in USA due to its major resistance to the major treatment methods [6]. Colon cancer is a complex disease whereby it involves a combination of both environmental and genetic factors. The process of colon cancer development requires several genetic factors that aid in the progression of benign adenoma to malignant carcinoma. Factors involved in colon cancer progression include: accumulation of chromosomal abnormalities, genetic mutations, and epigenetic changes leading to the inhibition of tumor suppressor genes and DNA mismatch repair genes or the stimulation of oncogenes [7-9].
Colon cancer is characterized by the development of adenomatous polyps and malignant cells in the colon. These abnormal cells producing tumors are characterized by uncontrolled replication and the property of metastasis. Moreover, colon cancer may develop due to the accumulation of molecular changes such as mutations in Kirsten-ras, p53, and adenomatous polyposis coli [10, 11]. There are several factors implicated in the prognosis of colon cancer that include the stage of disease, site of metastasis, type of treatment given and tumor genetic mutations [12]. Despite advances in understanding the pathogenesis, diagnosis, and treatment of colon cancer, it remains lethal to the patient especially if discovered at later stages [13].
Angiogenesis, the hallmark of cancer pathogenesis and metastasis and an attractive target for antitumor therapies, is a complex mechanism characterized by the formation of new vessels from pre-existing blood vessels [13, 14]. This complex process involves multiple signaling pathways that include proangiogenic and antiangiogenic factors, extracellular matrix components, and cell types, thus impacting the type and location of the angiogenic response [14]. The most prominent among these in promoting angiogenesis is the pathway involving Vascular Endothelial Growth Factor (VEGF) signalling molecule and its cognate receptor (VEGF receptor 2 (VEGFR-2). This pathway is highly expressed in human cancers leading to the formation and branching of new tumor blood vessels, the development of rapid tumor growth, and the metastatic potential of tumor cells. VEGF consists of a family of ligands that include VEGF-A to -D and Placental Growth Factor (PlGF) ligands that bind to the VEGFR tyrosine kinase receptors [14, 15]. Among those, VEGF-A and VEGF-B have a significant role in angiogenesis through their greatest binding affinity for VEGFR-1 and VEGFR-2. Initial anticancer clinical treatments aimed to inhibit VEGF/VEGFR signalling pathway and therefore, several VEGF/VEGFR targeted inhibitors such as monoclonal antibodies and fusion proteins were developed and approved to treat and improve the prognosis of cancer [13,14]. On the other hand, there are several oncogenes known to be over-expressed in colon cancer such as the Kirsten Rat Sarcoma Viral Oncogene Homolog (KRAS), proto-oncogene B-Raf and v-Raf murine sarcoma viral oncogene homolog B (BRAF), and Phosphoinositide 3-kinases (PI3K) which normally stimulate the cell to divide in response to growth factors. Mutation in these genes results in over-activation of cell proliferation [8, 16-18]. Moreover, several protein kinases have been involved in pathophysiological conditions like cancer including the mechanistic Target of Rapamycin (mTOR) and protein kinase B (Akt) hence rendering them a significant target to cancer therapeutic research [19-21]. mTOR, an atypical protein kinase that belongs to PI3K-related kinase family, is known to inhibit autophagy and enhance cell growth [20]. Akt, a serine-threonine protein kinase, plays a significant role in inhibiting apoptosis and enhancing cell growth and angiogenesis.
Treatment of colon cancer includes chemotherapy, radiation, surgery, and other specifically targeted therapies like immunotherapy. Surgery is the main used treatment until now; however, immunotherapy can be used as an adjunct to surgery [22]. In the revolution of understanding the interaction between tumor and immune cells, the development of antitumor therapeutic agents using immune cells has been advanced [23,24]. The immune system has played a critical yet dual role in suppressing or promoting cancer development resulting in cancer immunoediting. The immunoediting process consists of 3 phases: elimination, equilibrium, and escape. CD8+ T cells played a significant role in cancer immunoediting [25]. Adoptive Cell Transfer (ACT) is an advanced and most effective type of immunotherapy in treating cancer whereby the natural ability of T cells to fight cancer is boosted. ACT was first developed using tumor-infiltrating lymphocytes (TILs) whereby TILs from the cancer patients are isolated, expanded in vitro using a high concentration of IL-2, and then injected back into the same patients [22,26-29]. A recent study conducted on 16 patients with metastatic gastrointestinal cancer demonstrated that naturally occurring cytotoxic CD8+ T cells can specifically react against metastatic gastrointestinal cancers. These results opened a door for the development of T-cell based immunotherapies in gastrointestinal cancer treatment while taking into consideration the challenges that were faced including the low percentages of tumor specific CD8 T cells [30]. It was also shown that chemotherapy-induced lymphodepletion prior to ACT activates the persistence and anticancer effectiveness of the injected cells [23]. The tyrosine kinase inhibitor, Sorafenib, is one of the commonly used chemotherapeutic drugs in colon cancer treatment. Sorafenib is known to inhibit tumor growth, angiogenesis, Mitogen-Activated Protein Kinase (MAPK) signaling pathways, VEGFR, Platelet-Derived Growth Factor Receptor (PDGFR) and Raf family kinases inducing autophagy of tumor cells [31-34].
The aim of this study is to determine the effect of ACT alone or in combination with the chemotherapeutic agent, Sorafenib, on angiogenesis, specifically VEGF/VEGFR pathway, and certain oncogenes and protein kinases (KRAS, BRAF, Akt, mTOR and PI3K) in DMH-induced colon cancer in Balb/c mice. This is the first study to target ACT in colon cancer in vivo that studies the effect of ACT on VEGF/VEGFR angiogenesis pathway.
Subject and Methods
Chemicals
1,2 Dimethylhydrazine dihydrochloride (DMH) was obtained from ACROS Organics TM (Thermo Fisher Scientific, USA). Sorafenib Tosylate was purchased from Eton Bioscience Inc. (catalogue number 1100200013). All primers were purchased from Macrogen (South Korea). Mojosort Mouse CD8 T cell Isolation Kit (catalogue number 480035), Mojosort Buffer 5 x (catalogue number 480017), Mojosort Magnet (catalogue number 480020), purified anti-mouse CD3 (catalogue number 100301), Purified anti-mouse CD28 (catalogue number 102101), Recombinant Mouse IL-7 (catalogue number 577804), Recombinant Mouse IL-2 (catalogue number 714604), anti-CD8-APC (clone 53-6.7), anti-CD4-FITC (clone GK 1.5), and anti-CD19-PerCP/Cyanine5.5 (clone 1D3) were obtained from BioLegend, USA. L-glutamine (catalogue number R8758), fetal bovine serum (catalogue number F9665), 2 mercaptoethanol (catalogue number M6250), and 100 x ITS liquid media supplement (I3146-5 mL) were purchased from Sigma Aldrich (USA). 100 x penicillin-streptomycin solutions (catalogue number L0022-100) were purchased from Biowest (USA). Fixable viability dye (eFluor 506) was obtained from eBiosciences (USA). Direct-zol RNA Miniprep (Catalogue number: R2051) was purchased from Zymo Research, USA to be used for RNA extraction. QuantiTect® Reverse Transcription Kit (catalogue number: 205311) was obtained from QIAGEN®, USA to be used for reverse transcription. 5 x HOT FIREPol® EvaGreen qPCR Mix Plus (no ROX) (catalogue number: 08-25- 00001) was obtained from Solis BioDyne, Estonia for RT-PCR. All other chemicals used were of high analytical grades.
Animal model
Healthy 6-week-old female albino Balb/c mice were obtained from Beirut Arab University's animal facility (18-20 g each). Mice were placed under standard laboratory conditions of light (12-hour light/dark cycle), temperature (22 ± 2ºC), and humidity with ad libitum access to standard mouse diet and tap water. They were left to adapt under these conditions for one week prior to starting the experiments. Experimental procedures were carried according to the approved guidelines of the Institutional Review Board (IRB) at Beirut Arab University.
Colon cancer induction
A total of 36 female Balb/c mice were used in this study. Mice were grouped 6 per cage. One group was used as normal control. The other mice were intraperitoneally injected with 1,2 dimethylhydrazine at 20 mg/kg body weight over a period of 12 consecutive weeks to induce colorectal cancer (Saxena et al. 2017). One week after the last DMH injection, 6 mice were fasted overnight, then sacrificed and their spleens were excised to isolate CD8+ T cells.
Histopathological testing
After dissection, the entire large intestine from the ileocecal junction to the anal verge were gently excised, opened longitudinally, flushed with ice-cold PBS, and the number of grown colorectal tumors was counted under macroscopic inspection by two observers. Part of the colorectal tissue was directly fixed in 10% formalin at room temperature for 24 hr and sent to Specialized Medical Laboratories (Beirut, Lebanon) for histopathological examination using Hematoxylin and Eosin (H and E) staining.
Spleen/Lymph node harvesting and CD8 T cell Isolation
Spleens in addition to inguinal, mesenteric and axiliary lymph nodes were separated from the DMH injected Balb/c mice. Tissues were harvested in vitro and homogenized to release splenocytes and lymph node cells, respectively in 5 mL RPMI 1640 medium. The cell suspension was centrifuged for 5 min at 400 g at room temperature. The cell pellet was resuspended in 900 μL of sterile water, 100 μL of 10 × PBS and 5 ml of serum-free RPMI medium. The single-cell suspensions isolated were from these tissues were pooled, filtered using a 40 μm cell strainer (Fisher), and counted (Lewis et al. 2015). Splenic and lymph node CD8+ T cells were isolated and enriched by negative selection using Mojosort Mouse CD8 T cell Isolation Kit, Mojosort Buffer 5 x and Mojosort Magnet according to manufacturer’s protocol. To assess sample purity, cell samples were taken prior to and after magnetic selection for staining using a fixable viability dye, anti-CD8-APC, anti-CD4-FITC and anti-CD19-PerCP/Cyanine5.5. Negative selection of CD8+ T cells gave a 92% pure CD8 T cell population (n=6) with 1.2% of CD4 T cell contamination and 2.21% of B cell contamination and a very high viability (<99%).
Cytotoxic (CD8) T cell culture in vitro and Adoptive CD8 T cell Therapy
CD8 T cells were plated into 6-well plates (Thermo Fisher Scientific) coated with 0.5 μg/mL purified anti-mouse CD3 and 5 μg/mL Purified anti-mouse CD28 in PBS (5 mL/well) overnight at 4ºC. The CD8+ T cells were cultured on day 0 at 5 x 106 per well in RPMI 1640 medium with L-glutamine, 10% heat-activated fetal bovine serum, 50 U/mL of penicillinstreptomycin solution, 50 μM of 2-mercaptoethanol, and 1% of 100 x ITS liquid media supplement. After incubation for 24 hrs at 37ºC, 0.5 ng/mL of Recombinant Mouse IL-7 and 30 U/ mL of Recombinant Mouse IL-2 were added to the cells and re-cultured for additional 24 hrs. After that, cells were harvested from the wells, resuspended in the medium, subcultured in a 6-well plate at 1 × 106 cells/5 ml/well in the presence of fresh medium containing all supplements and IL-2 and IL-7. After incubation for 24 hrs, cells were harvested and replated for additional 24 hrs as mentioned earlier. On day four, cells were harvested and used for adoptive transfer 2 weeks following the last DMH injection as shown in (Table 1). Recipient mice received the cells by injection in the tail vein at 10 × 106 cells per mouse. Sorafenib treatment was given by gavage as was the DMSO vehicle used to dissolve the sorafenib.
Tab. 1. Experimental Design
| Group A: Normal Control | Mice didn’t receive any type of injections throughout the experiment. This group is referred to as a normal control. |
| Group B: DMH-injected | Mice received DMH dissolved in saline (20 mg/Kg; intraperitoneal injection) once per week over 12 weeks to induce colorectal cancer (CRC). This group was sacrificed 1 week after the last DMH injection to isolate the tumor specific T cells from the spleens and lymphnodes. |
| Group C: DMSO | Mice received DMSO (1% DMSO in PBS) by gavage for 2 weeks after the final DMH exposure and continued for 5 consecutive days. DMSO was used as a vehicle to dissolve sorafenib. |
| Group D: Sorafenib Treatment | Mice received Sorafenib Tosylate treatment at 30 mg/kg by gavage 2 weeks after the final DMH exposure and continued for 5 consecutive days. Sorafenib is used as a chemotherapeutic drug to treat colon cancer. |
| Group E: CD8 T cell Treatment | Mice received tail IV injections of CD8 T cells at 10 × 106 cells per mouse starting 2 weeks after the final DMH injection and continued till 5 consecutive days. CD8 T cell therapy is referred to as the Adoptive immunotherapeutic treatment. |
| Group F: Sorafenib+CD8 T cell Treatment | Mice received oral Sorafenib Tosylate treatment at 30 mg/kg in combination with CD8 T cell IV injections at 10 × 106 cells per mouse for 5 consecutive days 2 weeks after the final DMH injection. This is referred to as the combination of chemotherapeutic and immunotherapeutic treatment. |
Experimental design
Two weeks following the last DMH injection to induce colon cancer, DMH mice were randomly divided into 4 experimental groups of 6 mice each as shown in Table 1. Mice belonging to Group A were normal healthy mice used as control.
Quantification of signaling gene expressions by RT-PCR
RNA extraction and quantification: Total RNA was extracted from colon homogenates using the Direct-zol RNA Miniprep according to the manufacturer’s protocol. Briefly, tissues were homogenized in 600 μl of TRI reagent and centrifuged at 10,000- 16,000 g for 30 seconds to collect the supernatant. An equal of volume of ethanol (95-100%) was added to the supernatant and transferred into Zymo-Spin IIC Column with 400 μl RNA wash buffer in a collection tube. Further to centrifugation, 5 μl DNase I, 75 μl DNA Digestion Buffer were mixed in RNase free tube, added to the column matrix and incubated at room temperature for 15 minutes. Further to incubation, 400 μl of Direct-zol Wash Buffer was added to the column and centrifuged to collect the pellet; this step was repeated twice. After that, 700 μl of RNA wash buffer was added to the column and centrifuged for 2 minutes. Then, column was transferred into an RNase-free tube where 50 μlof DNase/RNase Free Water were added to the column matrix and centrifuged to elute the RNA. To check for the integrity of the eluted RNA, samples were electrophoretically separated on 1% agarose and visualized by UV illumination using ethidium bromide staining (results not shown). RNAs appeared as two sharp bands corresponding to the 28 S rRNA and 18 S rRNA. RNA was quantified using DeNovix (Blue) DS- 11 Spectrophotometer (DNA-RNA Quantification) through its absorbance which was measured at 260 nm. Its purity was assessed from the 260/280 absorbance ratio.
Reverse transcription: RNA was transcribed using the QuantiTect® Reverse Transcription Kit according to manufacturer’s protocol. Briefly, 1 μg of RNA samples were mixed with 3 μL gDNA Wipeout Buffer and 9 μL of RNase free water and incubated at 42ºC for 2 minutes to effectively remove any contaminated genomic DNA in a total volume of 14 μL. Following genomic DNA removal, RNA samples were reverse transcribed using Quantiscript Reverse Transcriptase (1.5 μ l), Quantiscript RT Buffer (6 μl), and RT Primer Mix (1.5 μl) at 42ºC for 15 minutes in a final volume of 20 μl. The enzyme was then inactivated at 95ºC for 3 minutes and the cDNA obtained was stored at -70ºC for later use.
RT-PCR: The expression of VEGF and VEGFR signaling genes and the expression of KRAS, BRAF, Akt, mTOR and PI3K were quantified by RT-PCR using 5 x HOT FIREPol® EvaGreen qPCR Mix Plus (no ROX). The amplification reaction was carried out at final volume of 20 μL containing 4 μL of 5 x HOT FIREPol® EvaGreen qPCR Mix Plus (1 x), 0.5 μL (10 pmol/μl; 250 nM) of each primer (forward and reverse, (Table 2), 3 μL of cDNA and 12 μL of H2O PCR grade. All primers (forward and reverse) were purchased from Macrogen, South Korea. An initial activation was performed at 95ºC for 12 minutes followed by 40 cycles of: 1. a denaturation step at 95ºC for 15 seconds; 2. an annealing step at 60ºC for 20 s; and 3. an elongation step at 72ºC for 20 seconds. Each RT-PCR was performed in triplicate for RT-PCR yield validation.
Tab. 2. The forward and reverse sequences of primers used to amplify the selected genes
| Gene Name | Sequence Direction | Sequence | References |
|---|---|---|---|
| GAPDH | F | 5’-TGGTGCTCAGTGTAGCCCAG-3’ | Basma et al. 2016 |
| R | 5’-GGACCTGACCTGCCGTCTAG-3’ | ||
| AKT1 | F | 5’- TCCTCAAGAACGATGGCACC -3’ | Eid et al. 2016 |
| R | 5’- CTCCTCAGGCGTTTCCACAT -3’ | ||
| PI3K | F | 5’- CTGGGGGACATACTGACTGT -3’ | Kuang et al. 2018 |
| R | 5’- GTTCCTGGAAAGTCTCCCCTC -3’ | ||
| mTOR | F | 5’- CTCAACATCGAGCATCGCATCATG -3’ | Fujishita et al. 2008 |
| R | 5’- ACCAGAAAGGGCACCAGC -3’ | ||
| KRAS | F | 5’-GATGCCTTCTATACATTAGTT-3’ | Chan et al. 2012 |
| R | 5’-AGGACTCTGAAGATGTACCTA-3’ | ||
| VEGFR-1 | F | 5’-CGGAAGGAAGACAGCTCATC-3’ | Lee et al. 2010 |
| R | 5’-CTTCACGCGACAGGTGTAGA-3’ | ||
| VEGFR-2 | F | 5’-GGCGGTGGTGACAGTATCTT-3’ | Lee et al. 2010 |
| R | 5’-TCTCCGGCAAGCTCAAT-3’ | ||
| VEGF-A | F | 5’-ACTTGTGTTGGGAGGAGGATGTC-3’ | Zadeh et al. 2019 |
| R | 5’-AATGGGTTTGTCGTGTTTCTGG-3’ | ||
| BRAF | F | 5’- GAAAGTGGCATGGTGATGTG -3’ | Walentinsson et al. 2001 |
| R | 5’- AGGTATCCTCGTCCCACCAT -3’ |
F=Forward set; R=Reverse set
Gene expression was measured by comparative threshold cycle (Ct) method using glyceraldehyde-3 phosphate dehydrogenase (GAPDH) as a reference gene. For each gene, the mean Ct (mCt) values were determined. ΔCt value was determined as the difference between the Ct of gene of interest and the Ct of GAPDH gene. The relative quantity of gene of interest expression compared to GAPDH gene was calculated applying the gene dosage ratio formula (GDR=2−ΔΔCCT) where: ΔΔCt=(mCt gene of interest-mCt GAPDH) control sample-(mCt gene of interest-mCt GAPDH) test sample.
Statistical analysis
All statistical analyses were performed using Microsoft Excel and SPSS 25, and they are shown as mean with standard deviations. Statistical significance was assessed using One-way ANOVA test followed by T-test. Graphs were drawn by GraphPad prism software and statistical significance was reported with a p-value <0.05 considered as significant.
Results
Histopathological results
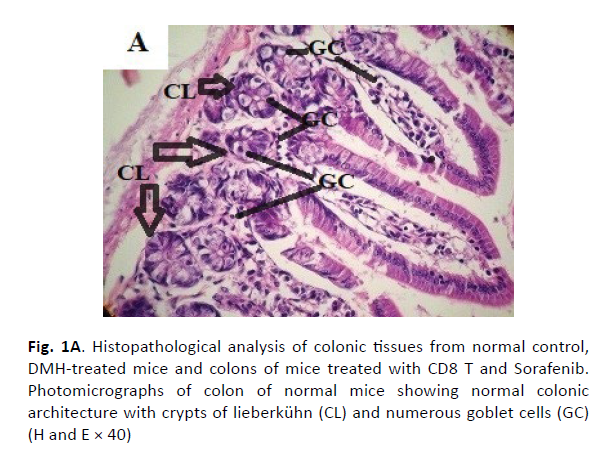
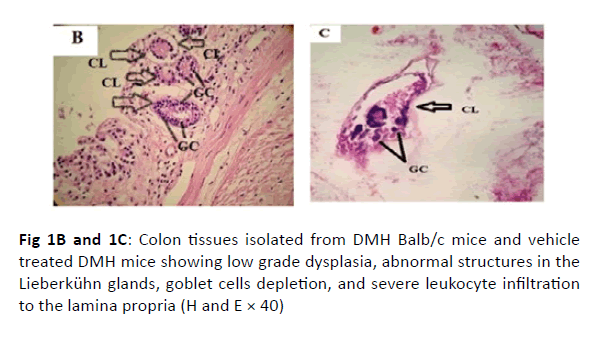
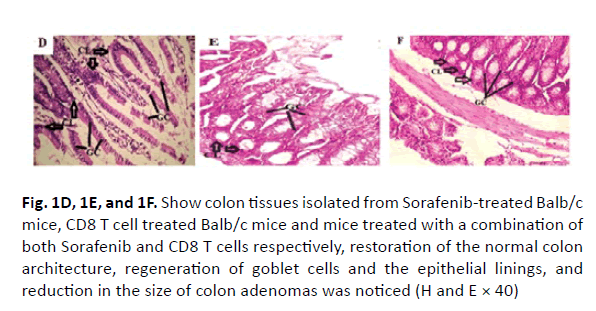
The colonic tissues of the healthy control mice showed normal histological architecture with their straight crypts of Lieberkuhn extending down to the muscularis mucosa in addition to the visible numerous goblet cells lining the crypts (Figure 1A). Histopathological study revealed the presence of polyps in the colons of group B mice that received DMH for 12 weeks as well as histological features of adenoma (Figure 1B). In this group, there were low grade dysplasia, abnormal structures in the Lieberkühn glands, goblet cells depletion, severe leukocyte infiltration to the lamina propria, and neoplastic cells formation of gland-like structures called lymphoid follicles accompanying cystic dilation. More pronounced damaging effects of DMH were noticed after 14 weeks in vehicle treated group (Group C) as shown in (Figure 1C). In this group, there were evident histological features of adenocarcinoma including high grade dysplasia, goblet cells depletion, leukocyte infiltration, and more prominent neoplastic invasion to the muscular layers of the intestine and formation of hyperplastic lymphoid follicles with cystic dilution. On the other hand, upon treatment of DMHinjected mice with sorafenib, CD8 T cells or a combination of both, the normal architecture of the colon was restored, the regeneration of goblet cells and epithelial linings was induced, and the size of colon adenomas was reduced (Figures 1D, 1E, and 1F, respectively).
Figure 1A: Histopathological analysis of colonic tissues from normal control, DMH-treated mice and colons of mice treated with CD8 T and Sorafenib. Photomicrographs of colon of normal mice showing normal colonic architecture with crypts of lieberkühn (CL) and numerous goblet cells (GC) (H and E × 40)
Figure 1B and 1C: Colon tissues isolated from DMH Balb/c mice and vehicle treated DMH mice showing low grade dysplasia, abnormal structures in the Lieberkühn glands, goblet cells depletion, and severe leukocyte infiltration to the lamina propria (H and E × 40)
Figure 1D, 1E, and 1F: Show colon tissues isolated from Sorafenib-treated Balb/c mice, CD8 T cell treated Balb/c mice and mice treated with a combination of both Sorafenib and CD8 T cells respectively, restoration of the normal colon architecture, regeneration of goblet cells and the epithelial linings, and reduction in the size of colon adenomas was noticed (H and E × 40)
Effect of Sorafenib and CD8 T cell treatment on Gene expression levels
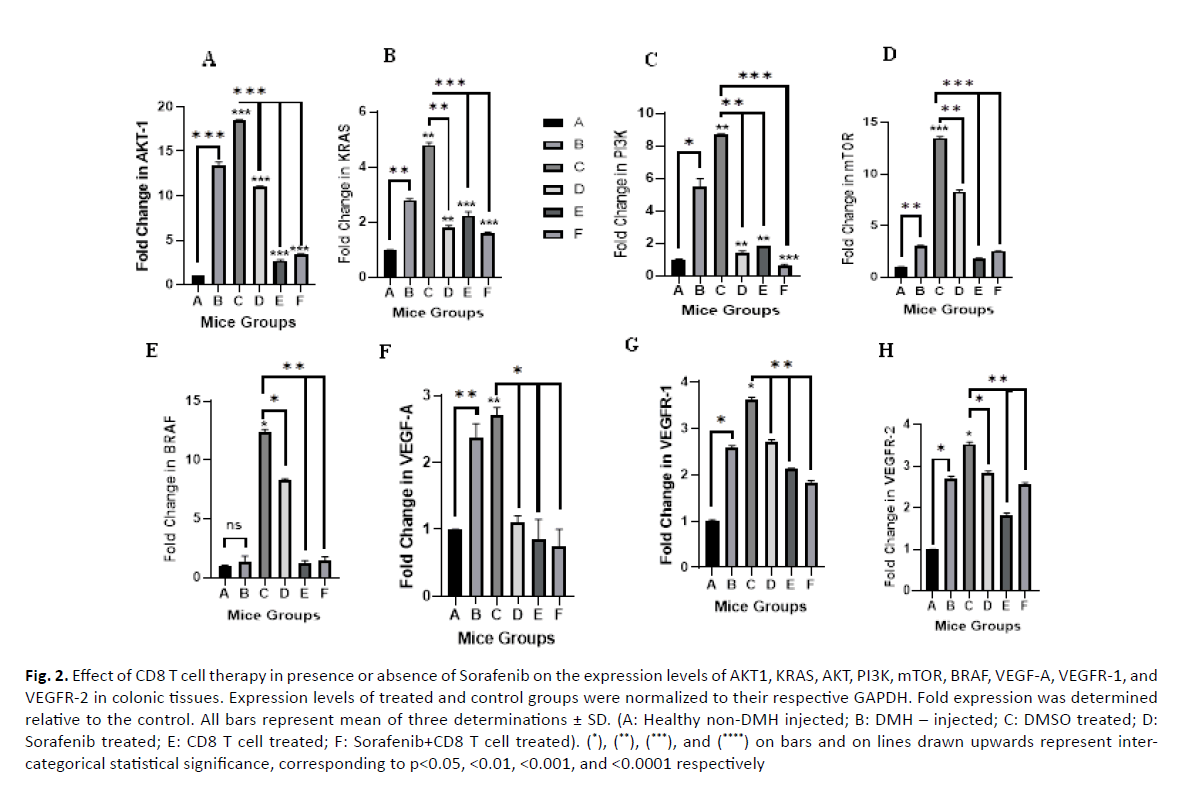
Effect of Sorafenib and CD8 T cell treatment on the expression of AKT1: After 12 weeks, DMH induced a significant increase in AKT1 expression by 13.8 folds (p value=0.0001) compared to the normal control Group A (Figure 2A). Similarly, further significant increase was observed in DMH mice treated with DMSO (Group C), the vehicle used to dissolve Sorafenib (18.47 fold, p value=0.0001). On the other hand, Sorafenib, CD8T and the combined treatment (Group D, E and F) induced a significant decrease in AKT expression by 7.4, 15.76, 15.06 folds with p values 0.0001, respectively compared to the DMSO Group C.
Figure 2: Effect of CD8 T cell therapy in presence or absence of Sorafenib on the expression levels of AKT1, KRAS, AKT, PI3K, mTOR, BRAF, VEGF-A, VEGFR-1, and VEGFR-2 in colonic tissues. Expression levels of treated and control groups were normalized to their respective GAPDH. Fold expression was determined relative to the control. All bars represent mean of three determinations ± SD. (A: Healthy non-DMH injected; B: DMH – injected; C: DMSO treated; D: Sorafenib treated; E: CD8 T cell treated; F: Sorafenib+CD8 T cell treated). (*), (**), (***), and (****) on bars and on lines drawn upwards represent intercategorical statistical significance, corresponding to p<0.05, <0.01, <0.001, and <0.0001 respectively
Effect of Sorafenib and CD8 T cell treatment on the expression of KRAS: DMH also induced a significant increase in KRAS expression (2.81 folds, p value=0.001) compared to control (Group B vs A) as shown in (Figure 2B). DMSO (Group C) enhanced further the effect of DMH and induced further significant increase in KRAS expression (4.79 folds, p value=0.001) compared to normal control. However, sorafenib treatment, CD8 T cell treatment and the combined treatment (Groups D, E and F) induced a significant decrease in KRAS expression by 3 (p value=0.002), 2.55 (p value=0.0001) and 3.2 (p value=0.0001) folds, respectively.
Effect of Sorafenib and CD8 T cell treatment on the expression of PI3K: The expression of PI3K significantly increased in DMH mice (Group B) and DMSO mice (Group C) by 5.5 and 8.72 folds (p values=0.012 and 0.004) respectively, compared to normal control group A. Sorafenib treatment, CD8 T cell treatment and the combined sorafenib and CD8 T cells treatment significantly decreased the expression of PI3K by 7.32 (p value=0.006), 6.9 (p value=0.001), and 8.12 folds (p value=0.0001), respectively compared to their control (Group C) as shown in (Figure 2C).
Effect of Sorafenib and CD8 T cell treatment on the expression of mTOR: Moreover, DMH was able to induce a significant increase in mTOR expression (3.03 folds, p value=0.001) compared to control group (Group B vs A) as shown in (Figure 2D). DMSO (Group C) enhanced further the effect of DMH and induced further significant increase in mTOR expression (13.48 folds, p value=0.0001) compared to normal control. However, sorafenib treatment, CD8 T cell treatment and the combined treatment (Groups D, E and F) induced a significant decrease in mTOR expression by 5.18 (p value=0.001), 11.68 (p value=0.0001) and 10.96 (p value=0.0001) folds, respectively. However, as we compared all groups to each other, there was a non-significant association since the p value was equivalent to 0.055.
Effect of Sorafenib and CD8 T cell treatment on the expression of BRAF: The expression of BRAF increased slightly by 1.37 folds (p value=0.346) in DMH injected mice (Group B) 12 weeks after the final DMH injection as compared to group A. This expression was further increased in vehicle injected mice (Group C) 14 weeks after the final DMH injection to 12.38 folds (p value=0.011) as compared to group A. Sorafenib treatment induced a slight yet significant decrease in the expression of BRAF as compared to vehicle injected group to 8.3 folds (p value=0.011). However, CD8 T cell treatment (Group E) and a treatment combining both sorafenib and CD8 T cells (Group F) were able to induce major reduction in the expression of BRAF to 1.18 (p value=0.001) and 1.44 folds (p value=0.001), respectively as compared to DMSO Group C (Figure 2E).
Effect of Sorafenib and CD8 T cell treatment on the expression of VEGF/VEGFR: DMH was able to increase the expression levels of VEGF-A, VEGFR-1 and VEGFR-2 by 2.38 (p value=0.002), 2.58 (p value=0.019) and 2.71 (p value=0.018) folds respectively as compared to control group (Group B vs. Group A). The expression levels of the 3 genes were slightly increased in DMSO mice (Group C) to 2.7 (p value=0.002), 3.62 (p value=0.012), and 3.53 (p value=0.012) folds, respectively as compared to group A. Sorafenib, CD8 T cell and combined treatment induced a slight, yet significant, decrease in the expression of VEGF-A of 1.1 fold (p value=0.042), 0.85 fold (p value=0.016) and 0.75 fold (p value=0.015), respectively (Figure 2F). For VEGFR-1, the fold decrease was 2.71 (p value=0.006), 2.12 (p value=0.009) and 1.82 (p value=0.002) folds, respectively (Figure 2G). As for the VEGFR-2 expression, the fold decrease was 2.84 (p value=0.010), 1.82 (p value=0.001), and 2.56 (p value=0.005) folds, respectively as compared to DMSO mice Group C as shown in (Figure 2H).
Discussion
Colon cancer is one the most common cancers worldwide and associated with high mortality rate [1, 2]. Further to chemotherapeutic resistance, cancer drug escape mechanisms and the side effects of chemotherapeutic drugs, the concept of immunotherapy was advanced [23]. Since it was shown that chemotherapy-induced lymphodepletion prior to ACT activates the persistence and anticancer effectiveness of the injected cells [29], we aimed to study the effect of Adoptive T cell therapy (CD8 T cell therapy) alone or in adjunct to Sorafenib on VEGF/ VEGFR signaling pathway through studying the expression levels of KRAS, BRAF, Akt, mTOR and PI3K, VEGF, VEGFR1 and VEGFR2 in DMH-induced colon cancer in Balb/c mice.
The pathway involving Vascular Endothelial Growth Factor (VEGF) signaling molecule and its cognate receptor (VEGF receptor 2 (VEGFR-2) is the most prominent angiogenetic pathway involved in the formation and branching of new tumor blood vessels, the development of rapid tumor growth, and the metastatic potential of tumor cells [14, 15]. VEGF-A and VEGF-B have a significant role in angiogenesis through their greatest binding affinity for VEGFR-1 and VEGFR-2. Upon binding of VEGF ligand to VEGFR, tyrosine kinase receptors, the latter will be activated via phosphorylation. The phosphorylation of VEGFR will result in the activation of KRAS which in turn activates 2 different pathways: KRAS/ BRAFMEK/ERK pathway and PI3K/AKT/mTOR pathway leading to tumor cell progression, growth and proliferation [35, 36]. Initial anticancer clinical treatments aimed to inhibit VEGF/VEGFR signalling pathway and therefore, several VEGF/ VEGFR targeted inhibitors such as monoclonal antibodies and fusion proteins were developed and approved to treat and improve the prognosis of cancer [13, 14].
In our study, the induction of colon cancer by DMH in Groups B and C showed significant up regulations in the expression levels of VEGF-A (2.38 and 2.7-fold, respectively), VEGFR-1 (2.58 and 3.62- fold, respectively), and VEGFR-2 (2.71 and 3.53-fold, respectively compared to normal non-DMH treated mice (Group A); our results are consistent with previous studies [13,14,37]. Sorafenib treatment modulated the altered expression levels of VEGF-A (1.1), VEGFR-1 (2.71), and VEGFR-2 (2.84) genes compared to vehicle injected group 14 weeks after the final DMH injection as was proposed in previous studies [38,39]. It was proven by Nan Li et al. that Sorafenib administration in rats resulted in potential control of tumor growth and increased the survival of rats by inhibiting VEGF/VEGF-receptor expression and reducing tumor angiogenesis [39]. Similarly, a study conducted on patients with hepatocellular carcinoma showed that Sorafenib induced a decrease in VEGF/VEGFR expression and therefore, improving the overall patients’ survival [40]. In different studies, the effect of monoclonal antibody therapy, a type of immunotherapy, on VEGF/VEGFR signaling pathway was assessed. It was noticed that targeting VEGF and its receptor improved the perfusion of tissues, increased the numbers of Intratumoral effector T cells, and reduced the accumulations of immunosuppressive regulatory T cells in Renal Cell Carcinoma [39]. The effect of CD8 T cell therapy on VEGF/VEGFR pathway in colon cancer was not investigated in previous studies. In the current study, we show that CD8 T cell therapy alone or in presence of Sorafenib resulted in better outcome as compared to mere Sorafenib treatment in terms of VEGF-A, VEGFR-1 and VEGFR-2 expression levels.
PI3K/AKT/mTOR signaling pathway has also gotten a major focus in various types of cancers (colon, bone, brain, lung, breast, renal, endocrine tissue, and gastric cancers) due its significant role in regulating metabolism, growth, apoptosis, proliferation, survival, and angiogenesis [41-45]. Researchers proposed the importance of using this signaling pathway as a target for treating tumors and other related diseases [45,46]. Researchers also advanced that inhibiting this pathway may be a potential target for treating, inducing cancer cell apoptosis, and inhibiting metastasis in patients with colon cancer [46-51]. This pathway is known to be over-expressed in patients with colon cancer and aids in cancer progression via the transformation of normal cells into cancer cells [48, 52, 53]. This is consistent with our results where cancer induction was associated with an increase in the expression levels of PI3K, AKT, and mTOR by 5.5, 13.8 and 3.03 folds, respectively as compared to normal controls. Sorafenib administration aided in decreasing the expression of those genes to 1.4, 11.07 and 1.8 folds, respectively. However; CD8 T cell treatment in presence or absence of Sorafenib resulted in better outcome and further decrease and inhibition in the expression of these three genes. Although this is the first study to target the effect of CD8 T cell therapy on PI3K/ AKT/mTOR signaling pathway in colon cancer, our results are consistent with previous ones regarding the effect of Sorafenib treatment on this pathway in various types of cancers, especially hepatocellular carcinoma [37,54,55].
KRAS/BRAF/MEK/ERK pathway is one of the significant pathways described in colon cancer progression and treatment [56]. In our results, colon cancer induction increased the expression levels of BRAF and KRAS by 1.37 and 2.81 folds, respectively as compared to normal controls. These results were consistent with previous studies [56-58]. As was mentioned in previous studies and shown in our current study, Sorafenib treatment decreases and inhibits the expression of BRAF and KRAS in colon cancer [59,60]. Although several studies described the role of Sorafenib in decreasing KRAS expression, it was proposed that possibly the increased phosphorylation of AKT and the existing cross-talk between the PI3K/AKT and RAS/ERK axis may lead to Sorafenib resistance [60]. A previous study on patient with lung cancer revealed that the infusion of CD8+ cells targeting KRAS mediated effective antitumor immunotherapy against cancer [61]. Moreover, in patients with metastatic melanoma, BRAF inhibitors resulted in dramatic increase in tumor-infiltrating CD8+ T cells [62].
Conclusion
These proposed studies drew our attention to investigate the role of KRAS/BRAF pathway as a therapeutic target to adoptive T cell therapy in treating colon cancer. Our results revealed that further reduction in the expression levels of both KRAS and BRAF upon CD8 T cell treatment alone or in combination with Sorafenib as compared to pure chemotherapeutic treatment. In conclusion, our findings may open up future work on the effect CD8 T cell therapy in colon cancer especially in terms of producing new anti-colon cancer therapeutic agents targeting these pathways.
References
- Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, et al. Colorectal cancer. Lancet. 2010;375:1030-1047.
- Spallanzani A, Gelsomino F, Caputo F, Santini C, Andrikou K, et al. Immunotherapy in the treatment of colorectal cancer: a new kid on the block. J Can Meta Treat. 2018;4:28.
- Jia Y, Xu G, Zhou W, Wang Z, Meng L, et al. Diabetes promotes DMH-induced colorectal cancer by increasing the activity of glycolytic enzymes in rats. PloS one. 2014;9:e110455.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7-30.
- Issa IA, Noureddine M. Colorectal cancer screening: An updated review of the available options. World J Gastroenterol. 2017;23:5086-5096.
- Wang X, Zhang H, Chen X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019;2:141-160.
- Testa U, Pelosi E, Castelli G. Colorectal cancer: genetic abnormalities, tumor progression, tumor heterogeneity, clonal evolution and tumor-initiating cells. Med Sci (Basel). 2018;6:31.
- Korphaisarn K, Pongpaibul A, Roothumnong E, Pongsuktavorn K, Thamlikitkul L, et al. High Frequency of KRAS Codon 146 and FBXW7 Mutations in Thai Patients with Stage II-III Colon Cancer. Asian Pac J Cancer Prev. 2019;20:2319- 2326.
- Grady WM, Carethers JM. Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology. 2008;135:1079-1099.
- Mardi K, Sharma M, Bhardwaj M, Rao M. p53 expression in colorectal carcinomas and its correlation with clinicopathological parameters. Clin Can Invest J. 2017;6:26.
- Tiwari A, Saraf S, Verma A, Panda PK, Jain SK. Novel targeting approaches and signaling pathways of colorectal cancer: An insight. World J Gastroenterol. 2018;24:4428-4435.
- Bittoni A, Sotte V, Meletani T, Cantini L, Giampieri R, et al. Immunotherapy in colorectal cancer treatment: actual landscape and future perspectives. J Cancer Metastasis Treat. 2018;4:55.
- Zhao M, Yu Z, Li Z, Tang J, Lai X, et al. Expression of angiogenic growth factors VEGF, bFGF and ANG1 in colon cancer after bevacizumab treatment in vitro: A potential self-regulating mechanism. Oncol Rep. 2017;37:601-607.
- Zhao Y, Adjei AA. Targeting Angiogenesis in Cancer Therapy: Moving Beyond Vascular Endothelial Growth Factor. Oncologist. 2015;20:660-673.
- Radhakrishnan E, Bava SV, Narayanan SS, Nath LR, Thulasidasan AKT, et al. [6]-Gingerol induces caspase-dependent apoptosis and prevents PMA-induced proliferation in colon cancer cells by inhibiting MAPK/AP-1 signaling. PloS one. 2014; 9:e104401.
- Perše M, Cerar A. Morphological and molecular alterations in 1, 2 dimethylhydrazine and azoxymethane induced colon carcinogenesis in rats. J Biomed Biotechnol. 2011;2011:473964.
- Chakravarthi S, Krishnan B, Madhavan M, BK SC, Apoptosis and expression of p53 in colorectal neoplasms. Indian J Med Res. 1999; 111:95-102.
- Rao CV, Yamada HY. Genomic instability and colon carcinogenesis: from the perspective of genes. Front Oncol. 2013;3:130.
- Vadlakonda L, Pasupuleti M, Reddanna P. Role of PI3K-AKT-mTOR and Wnt Signaling Pathways in Transition of G1-S Phase of Cell Cycle in Cancer Cells. Front Oncol. 2013;3:85.
- Hua H, Kong Q, Zhang H, Wang J, Luo T, et al. Targeting mTOR for cancer therapy. J Hematol Oncol. 2019;12:71.
- Nitulescu GM, Van De Venter M, Nitulescu G, Ungurianu A, Juzenas P, et al. The Akt pathway in oncology therapy and beyond. Int J Oncol. 2018;53:2319-2331.
- Boghossian S, Robinson S, Von Delwig A, Manas D, White S. Immunotherapy for treating metastatic colorectal cancer. Surg Oncol. 2012;21:67-77.
- Wang M, Yin B, Wang HY, Wang RF. Current advances in T-cell-based cancer immunotherapy. Immunotherapy. 2014;6:1265-1278.
- Olson A, Li Y, Lin Y, Liu ET, Patnaik A. Mouse Models for Cancer Immunotherapy Research. Cancer Discov. 2018;8:1358-1365.
- Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest. 2015;125:3335-3337.
- Wang ZX, Cao JX, Liu ZP, Cui YX, Li CY, et al. Combination of chemotherapy and immunotherapy for colon cancer in China: a meta-analysis. World J Gastroenterol. 2014;20:1095-1106.
- Beatty JD. Immunotherapy of colorectal cancer. Cancer. 1992;70:1425-1433.
- Dittrich A, Kosty M, Jezdic S, Pyle D, Berardi R, et al. ESMO/ASCO recommendations for a global curriculum in medical oncology edition 2016. ESMO Open. 2016;1: e000097.
- Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909-915.
- Turcotte S, Gros A, Tran E, Lee CCR, Wunderlich JR, et al. Tumor-reactive CD8+ T cells in metastatic gastrointestinal cancer refractory to chemotherapy, Clin Cancer Res. 2014;20:331-343.
- Inoshita S, Takeda K, Hatai T, Terada Y, Sano M, et al. Phosphorylation and inactivation of myeloid cell leukemia 1 by JNK in response to oxidative stress. J Biol Chem. 2002;277:43730-43734.
- Panaretakis T, Laane E, Pokrovskaja K, Björklund AC, Moustakas A, et al. Doxorubicin requires the sequential activation of caspase-2, protein kinase Cdelta, and c-Jun NH2-terminal kinase to induce apoptosis. Mol Biol Cell. 2005;16:3821-3831.
- Abdel-Maksoud MS, Kim MR, El-Gamal MI, El-Din MMG, Tae J, et al. Design, synthesis, in vitro antiproliferative evaluation, and kinase inhibitory effects of a new series of imidazo [2, 1-b] thiazole derivatives. Eur J Med Chem. 2015;95:453-463.
- Kacan T, Nayir E, Altun A, Kilickap S, Babacan NA, et al. Antitumor activity of sorafenib on colorectal cancer. J Oncol Sci. 2016;2:53-57.
- Mohseni M, Park BH. PIK3CA and KRAS mutations predict for response to everolimus therapy: now that’s RAD001. J Clin Investig. 2010;120:2655-2658.
- Saletti P, Molinari F, De Dosso S, Frattini M. EGFR signaling in colorectal cancer: a clinical perspective. Gastrointest Cancer. 2015;5:21-38.
- Li A, Zhang R, Zhang Y, Liu X, Wang R, et al. BEZ235 increases sorafenib inhibition of hepatocellular carcinoma cells by suppressing the PI3K/AKT/mTOR pathway. Am J Transl Res. 2019;11:5573-5585.
- Huynh H, Ngo VC, Koong HN, Poon D, Choo SP, et al. Sorafenib and rapamycin induce growth suppression in mouse models of hepatocellular carcinoma. J Mol Cell Med. 2009;13:2673-2683.
- Li X, Shao C, Shi Y, Han W. Lessons learned from the blockade of immune checkpoints in cancer immunotherapy. J Hematol Oncol. 2018;11:1-26.
- Tsuchiya K, Asahina Y, Matsuda S, Muraoka M, Nakata T, et al. Changes in plasma vascular endothelial growth factor at 8 weeks after sorafenib administration as predictors of survival for advanced hepatocellular carcinoma. Cancer. 2014;120:229-237.
- Jiang N, Dai Q, Su X, Fu J, Feng X, et al. Role of PI3K/AKT pathway in cancer: the framework of malignant behavior. Mol Biol Rep. 2020; 47:4587-4629.
- Robbins HL, Hague A. The PI3K/Akt pathway in tumors of endocrine tissues. Front Endocrinol. 2016;6:188.
- Fang WL, Huang KH, Lan YT, Lin CH, Chang SC, et al. Mutations in PI3K/AKT pathway genes and amplifications of PIK3CA are associated with patterns of recurrence in gastric cancers. Oncotarget. 2016;7:6201-6220.
- Fan D, Liu Q, Wu F, Liu N, Qu H, et al. Prognostic significance of PI3K/AKT/mTOR signaling pathway members in clear cell renal cell carcinoma. Peer J. 2020;8:e9261.
- Xu F, Na L, Li Y, Chen L. Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell Biosci. 2020;10:1-2.
- You S, Li W, Guan Y. Tunicamycin inhibits colon carcinoma growth and aggressiveness via modulation of the ERK-JNK-mediated AKT/mTOR signaling pathway. Mol Med Rep. 2018;17:4203-4212.
- Ma JC, Sun XW, Su H, Chen Q, Guo TK, et al. Fibroblast-derived CXCL12/SDF-1α promotes CXCL6 secretion and co-operatively enhances metastatic potential through the PI3K/Akt/mTOR pathway in colon cancer. World J Gastroenterol. 2017;23:5167-5178.
- Ponnurangam S, Standing D, Rangarajan P, Subramaniam D. Tandutinib inhibits the Akt/mTOR signaling pathway to inhibit colon cancer growth. Mol Cancer Ther. 2013;12:598-609.
- Moench R, Grimmig T, Kannen V, Tripathi S, Faber M, et al. Exclusive inhibition of PI3K/Akt/mTOR signaling is not sufficient to prevent PDGF-mediated effects on glycolysis and proliferation in colorectal cancer. Oncotarget. 2016;7:68749-68767.
- Han C, Xing G, Zhang M, Zhong M, Han Z, et al. Wogonoside inhibits cell growth and induces mitochondrial-mediated autophagy-related apoptosis in human colon cancer cells through the PI3K/AKT/mTOR/p70S6K signaling pathway. Oncol Lett. 2018;15:4463-4470.
- Zhu ML, Zhang PM, Jiang M, Yu SW, Wang L. Myricetin induces apoptosis and autophagy by inhibiting PI3K/Akt/mTOR signalling in human colon cancer cells. BMC Complement Med Ther. 2020;20:209.
- Lian G, Chen S, Ouyang M, Li F, Chen L, et al. Colon cancer cell secretes EGF to promote M2 polarization of TAM through EGFR/PI3K/AKT/mTOR pathway. Technology in cancer research and treatment. 2019;18:1533033819849068.
- Tu FL, Guo XQ, Wu HX, He ZY, Wang F, et al. Circ-0001313/miRNA-510-5p/AKT2 axis promotes the development and progression of colon cancer. Am J Transl Res. 2020;12:281-291.
- Dai N, Ye R, He Q, Guo P, Chen H, et al. Capsaicin and sorafenib combination treatment exerts synergistic anti‑hepatocellular carcinoma activity by suppressing EGFR and PI3K/Akt/mTOR signaling. Oncol Rep. 2018;40:3235-3248.
- Singh AR, Joshi S, Burgoyne AM, Sicklick JK, Ikeda S, et al. Single agent and synergistic activity of the “first-in-class” dual PI3K/BRD4 inhibitor SF1126 with sorafenib in hepatocellular carcinoma. Mol Cancer Ther. 2016;15:2553-2562.
- McNew KL, Whipple WJ, Mehta AK, Grant TJ, Ray L, et al. MEK and TAK1 regulate apoptosis in colon cancer cells with KRAS-dependent activation of proinflammatory signaling. Mol Cancer Res. 2016;14:1204-1216.
- Bailey AM, Zhan L, Maru D, Shureiqi I, Pickering CR, et al. FXR silencing in human colon cancer by DNA methylation and KRAS signaling. Am J Physiol Gastrointest Liver Physiol. 2014;306:G48-G58.
- Wu DW, Lin PL, Cheng YW, Huang CC, Wang L, et al. DDX3 enhances oncogenic KRAS-induced tumor invasion in colorectal cancer via the β-catenin/ZEB1 axis. Oncotarget. 2016;7:22687-22699.
- Chen Y, Liu YC, Sung YC, Ramjiawan RR, Lin TT, et al. Overcoming sorafenib evasion in hepatocellular carcinoma using CXCR4-targeted nanoparticles to co-deliver MEK-inhibitors. Sci Rep. 2017;7:1-2.
- Fritsche-Guenther R, Witzel F, Kempa S, Brummer T, Sers C, et al. Effects of RAF inhibitors on PI3K/AKT signalling depend on mutational status of the RAS/RAF signalling axis. Oncotarget. 2016;7:7960-7969.
- Tran E, Robbins PF, Lu YC, Prickett TD, Gartner JJ, et al. T-cell transfer therapy targeting mutant KRAS in cancer. N Engl J Med. 2016;375:2255-2262.
- Cooper ZA, Frederick DT, Ahmed Z, Wargo JA. Combining checkpoint inhibitors and BRAF-targeted agents against metastatic melanoma. Oncoimmunol. 2013;2:e24320.