Review Article - Onkologia i Radioterapia ( 2023) Volume 17, Issue 9
Immunohistochemistry evaluation of CD40 in Iraqi patients with gastric cancer
Asraa Naduhm Abd-Alsatar1* and Israa A. Dheeb22Assistant Professor of Microbiology, College of Medicine, University of Al-Qadisiyah, Diwaniyah, Iraq
Asraa Naduhm Abd-Alsatar, Department of Nursing Techniques, Technical Institute of Al-Diwaniyah, AL-Furat AL Awsat Technical University, Iraq, Email: Asraa.alsatar@atu.edu.iq
Received: 25-Aug-2023, Manuscript No. OAR-23-111457; Accepted: 14-Sep-2023, Pre QC No. OAR-23-111457 (PQ); Editor assigned: 28-Aug-2023, Pre QC No. OAR-23-111457 (PQ); Reviewed: 03-Sep-2023, QC No. OAR-23-111457 (Q); Revised: 12-Sep-2023, Manuscript No. OAR-23-111457 (R); Published: 19-Aug-2023
Abstract
Background: CD40 is one of the most important cellular markers that involved in many immune reactions and pathological pathways. The present investigation purposes to examine the immune-histochemical expression of CD40 in Gastric Tumor (GC) patients in Iraq. Methods: Serum concentrations of CCL22 and CCL5 were determined in cases of GC after and before tumor eradication using ELISA kits, and the examination was carried out according to the manufacturer's instructions, while those indicators were determined in paraffin-embedded blocks of stomach tissues immunohistologically using the Immunohistochemistry (IHC) method. Results: Present study included 40 cases of gastric cancer. Age of the cases extended from 31 years to 78 years with an average about 55.3 years and most patients are males (60%). We found most of the patients in the fourth (70%) and third stages (25%), in which the cancer is considered to be in the advanced stage. IHC assay determine that CD40 are exceedingly articulated in gastric tumor cells. Serologically, CD40 was significantly higher (P<0.05) among cases before surgery (26.705 pg/ml) than after surgery (18.30 pg/ml) and controls (7.22 pg/ml). Current study found the level of CD40 evaluated in males compared to females but without statistical differences (P>0.05). Moreover, we found the highest concentration of CD40 after and before surgery in advanced metastatic cancer cases (stage IV). In Conclusion, we found that CD40 is histo-immunologically linked with GC, which may play a role in the progression and spread of GC. Therefore, CD40 can be relied upon in the diagnosis and treatment of GC.
Keywords
immunohistochemistry, CD40, gastric cancer, expression
Introduction
CD40 (109535) belongs to the TNFR (Tumor Necrosis Factor Receptor) group. The cytoplasmic area of CD40, although shorter, bears some resemblance to the preserved cytosolic death domain found in FAS (134637) besides TNFR1 (191190). When CD40bp is translated in vitro, it appears to have a molecular mass of approximately 64 kD [1].
CD40, a receptor protein, is located on the cell membrane and its genome is found on chromosome number 20. Cells that prompt CD40 also co-prompt the receptor of CD40L. Upon interaction between CD40 and CD40L, CD40 becomes activated and is the cell internalizing triggered receptor CD40 then cooperates with the growth mortification receptor aspect, initiating gesturing paths that ultimately lead to the activation of NF-kappaB [1,2]. The CD40/CD40L system is well-known for its involvement in human carcinogenesis, acting as a connection between inflammation and neoplasia. This system is expressed in various cell types, including platelets, endothelium, and immune cells [3]. Notably, the primary source of soluble are the platelets of CD40L (sCD40L). Platelets express CD40L when stimulated by a extensive area of platelet activators like thrombin. Consequently, activated platelets may contribute to the inflammatory path that goes to carcinogenesis of human. Experimental research has demonstrated the role of CD40L in both inflammation and neoplasia [3,4].
CD40, an associate of the growth necrosis aspect receptor (TNF-R) superfamily and a co-signaling molecule, has been identified as a significant contributor to the process of carcinogenesis [5]. Within the realm of tumor immunology, CD40, along with its ligand CD40L, plays critical roles in regulating immune responses by influencing the purposes of T cells and antigen present cells (APCs) [6]. The CD40-CD40L axis has been implicated in various physiological activities, including immune disorders, cardiovascular disease, cancer, and even Alzheimer's illness. Notably, upregulation of CD40 expression has been observed in multiple types of epithelial cancers [7]. In gastric tumor tissue, the appearance CD40 levels and CD40L are definitely linked with lymphatic metastasis and the TNM stage of the tumor. In the context of mouse renal cancer cells, CD40 activation triggers cellular killing effects through the activation of Fas. Importantly, CD40 signaling plays a pivotal role in motivating antigen appearance, undercoat both cytotoxic T cells and helper, and initiating a diverse array of the responses of inflammatory [8].
Immunohistochemistry is employed in the current study to evaluate the cellular expression of CD40 in Iraqi patients diagnosed with stomach cancer. The binding interaction between CD40 and its ligand CD40L on macrophages triggers the manufacture of growth necrosis alpha aspect (TNF-α), AntibodyDependent Cellular Cytotoxicity (ADCC), and nitric oxide synthase. Furthermore, the compulsory of CD40 to CD40L on macrophages and dendritic cells goes to the discharge of Interleukin 12 (IL-12), which plays a vital role in facilitating T cell and Natural Killer Anticancer (NK) cell-mediated effects [9]. CD40-CD40L Initiation on cells B supports cell T resistance and enhances the manufacture of antibody. Moreover, direct activation of CD40 may induce apoptosis in cancer cells [10].
Materials and Methods
Study design and data collection
The present research encompasses a case-control investigation conducted on a troop of 40 cases, comprising 16 females and 24 males, selected from multiple hospitals in Iraq. The study was carried out over a period spanning from March 2022 to January 2023. Additionally, a control group consisting of 40 apparently healthy individuals, including 21 males and 19 females, without any history of systemic disease, was included for comparison. The study adhered to the ethical guidelines of Al-Diwaniya City, and verbal learnt agreement was gotten from all contributors. The collected samples involved 3 ml of serum in plain tubes and paraffin-embedded stomach tissue.
Classification of gastric cancer
In the current study, the staging of gastric cancer was performed by a specialized doctor using the classification system established by Taniguchi et al. [11]. This system involves assigning numerical values to indicate the size of the tumor (ranging from 1 to 4), the involvement of lymph nodes (classified as N 0 to N3), a nd the presence of metastasis or spread of cancer (designated as M0 or M1). Lower numerical values indicate less advanced stages of cancer.
Immunological study
The p reparation o f r eagents a nd t he a ssay p rocedure w ere conducted in accordance with the manufacturer's instructions provided with the ELISA kits (Solarbio, China)Immunological study:
Immunohistochemistry assay
Previously paraffin-embedded block tissue samples were used to determine specific antigen of CD40. IHC protocol were performed according to manufacture's procedure using specific IHC kits (Solarbio / China).
Statistical analysis
The data were analyzed statistically using the Statistical Packages for Social Sciences version 19 program in addition to the Excel 2010 program, and the probability value smaller than 0.05 was considered to be significantly different.
Results
The present case-control investigation consisted of 40 cases diagnosed with gastric cancer, comprising 24 males and 16 females, as indicated in Table 1. Ranging age of the cases was from 31 years to 78 years, with a 55.3 years (SD ± 13.39). The control group comprised 40 apparently healthy individuals, including 21 males and 19 females, with ages ranging from 30 to 75 years and a 56.5 years as an average age (SD ± 11.66), as shown in Table 2. The majority of malignant cases were observed in the age group of 64 years to 78 years, accounting for 37.5% of the cases. This was followed by the age group of 42 years to 53 years, with a rate of 22.5%. Furthermore, it was found that males were more susceptible to gastric cancer, accounting for 60% of the cases, compared to females, who accounted for 40%.
Tab. 1. Distribution of patients with gastric cancer over age and gender
| Age Groups (years) | Males number | Females number | Total number (%) |
|---|---|---|---|
| 31-42 | 5 | 3 | 8 (20%) |
| 42-53 | 5 | 4 | 9 (22.5%) |
| 53 - 64 | 6 | 2 | 8 (20%) |
| 64-78 | 8 | 7 | 15 (37.5%) |
| Total | 24 (60%) | 16 (40%) | 40 |
Tab. 2. The case-control comparison according to the age mean.
| Age (years) | Healthy controls | Cases | P |
|---|---|---|---|
| Range | (30 - 75) | (31 - 78) | |
| Mean | 56.5 | 55.3 | 0.852 [NS] |
| SD | 11.66 | 13.39 | |
| SE | 1.844 | 2.117 | |
| Males (%) | 21 (52.5%) | 24 (60%) | 0.073 [NS] |
| Females (%) | 19 (47.5%) | 16 (40%) | 0.038[NS] |
| N. | 40 | 40 |
N=Number, NS=No Significant, SE=Standard Error, SD=Standard Deviation
Our study showed that most cases of stomach cancer in our society are diagnosed and treated after it reaches advanced stages, as we found most of the patients in the fourth (70%) and third stages (25%), in which the cancer is considered to be in the advanced stage, while we found only two patients in the second stage moreover we did not record any cases in the first stage as in Figure 1.
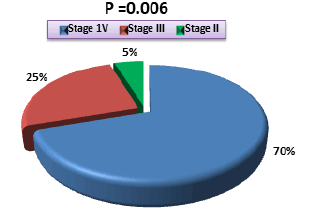
Figure 1: Frequency of gastric cancer stages
Immuno-histochemical analysis was conducted to examine the CD40 appearance in samples GC. The study also analyzed clinic-pathological factors such as age, sex, and stage in relation to the varying expression levels of CD40. The findings revealed a significant increase in CD40 expression within GC tissues, as depicted in Figure 2. The mean serum concentration of CD40 was significantly higher (p<0.05) among cases before surgery (26.705 pg/ml) than after surgery (18.30 pg/ml) and controls (7.22 pg/ml) as shown in Tables 3. It had been a noteworthy variances (p=0.047) in the concentration of CD40 after the surgery compared to the control.
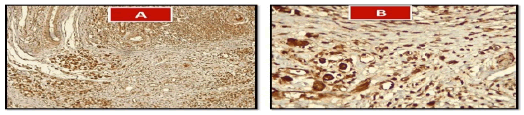
Figure 2: Section treated with CD 40 shows hyperchromatic malignant cells staining with chromogen in hematoxyline background (A;10X and B; 40 X)
Tab. 3. Comparison serum concentration of CD40 of studied groups
| Serum conc. pg/ml | Case-control comparison | P | |
|---|---|---|---|
| Before surgery | After surgery | Value | |
| CD40 | 0.0482 [S] | ||
| Range | 21.33 - 30.56 | 13.121 – 22.356 | |
| Mean | 26.705 | 18.3 | |
| SD | 4.396 | 2.457 | |
| SE | 0.695 | 0.388 | |
| Before surgery | Control | ||
| Range | 21.33 - 30.56 | 6.820 – 9.77 | |
| Mean | 26.705 | 7.22 | 0.0044 [S] |
| SD | 4.396 | 2.03 | |
| SE | 0.695 | 0.322 | |
| CD40 | After surgery | Control | 0.047 [S] |
| Range | 13.121 – 22.356 | 6.820 – 9.77 | |
| Mean | 18.3 | 7.22 | |
| SD | 2.457 | 2.03 | |
| SE | 0.388 | 0.322 | |
| Total number | 40 | 40 |
S: significant associations (P<0.05); NS: non-significant association (P>0.05); SD: standard deviation; SE: standard error
Our results showed that the concentrations of immunological indicators are affected by age, as we found the highest concentrations of CD40 (33.81 pg/ml) were in the elderly within the age group of 64 years - 78 years before surgery. Where we found clear differences (p<0.05) when distributing CD40 concentrations according to age groups as shown in Table 4. After surgery, we found the highest concentrations of CD40 (19.21 pg/ml) in patients in age group 64 years – 78 years. However, we did not find clear differences (p>0.05) when distributing the concentration of CD40according to age after surgery. Post or pre surgery, the level of CD40 evaluated in males compared to females but without statistical differences (p>0.05) as observed in Table 5.
Tab 4. Mean of serum concentration of CD40 according to patients age groups
| CD40 (pg/ml) | Age (years) Groups | X2 | P value | |||
|---|---|---|---|---|---|---|
| 31-42 | 42-53 | 53 - 64 | 64-78 | |||
| Before surgery | 22.6 | 24.11 | 25.11 | 33.81 | 3.95 | 0.04 [S] |
| After surgery | 18.22 | 13.55 | 15.01 | 19.21 | 0.25 | 0.416 [NS] |
| P value | 0.449 [NS] | 0.039 [S] | 0.035 [S] | 0.013 [S] |
S: significant associations (P<0.05); NS: non-significant association (P>0.05)
Tab 5. Mean of serum concentration of CD40 according to patients gender
| CD40 (pg/ml) | Females | Males | X2 | P value |
|---|---|---|---|---|
| Before surgery | 24.11 | 33.81 | 3.95 | 0.04 [NS] |
| After surgery | 18.11 | 19.06 | 0.116 | 0.411 [NS] |
| P value | 0.044 [S] | 0.043 [S] |
S: significant associations (P<0.05); NS: non-significant association (P>0.05)
In Table 6, we found a high concentration of CD40 after and before surgery in advanced metastatic cancer cases (stage IV), where the level before surgery were 29.71 pg/ml and after surgery 22.00 pg/ml.
Tab 6. Mean of serum concentration of CD40 according to cancer stages
| CD40 (pg/ml) |
cancer stages |
P value | ||
|---|---|---|---|---|
| Stage 2 | Stage 3 | Stage 4 | ||
| Before surgery | 21.4 | 27.43 | 29.71 | 0.411 [NS] |
| After surgery | 15.12 | 17.12 | 22 | 0.309 [NS] |
| P value | 0.166 [NS] | 0.043 [S] | 0.222 [NS] | |
| N | 40 | 40 | 40 |
Discussion
The cancer stage at the time of diagnosis refers to the extent of cancer within the body. It plays a crucial role in guiding treatment decisions and has a significant impact on the overall survival rate. It is important to note that the assigned stage numbers (ranging from stage 1 to 4) can change over time if the cancer exhibits growth, spreads to other parts of the body, or recurs after treatment [12]. These changes in staging reflect the evolving nature of the disease and the need for ongoing monitoring and adjustment of treatment approaches.
Our study findings revealed a concerning trend in the Iraqi society, where the majority of GC were diagnosed at advanced and widespread stages. Surprisingly, no patients were identified in the early stage of cancer. This observation may be attributed to the underestimation of early screening for cancer detection and a lack of awareness regarding the significance of early detection methods such as endoscopes or biopsies [13]. Another study conducted by Attia et al. reported that Grade II adenocarcinoma was the most frequently observed histological type in stomach cancer cases. Additionally, according to the Laurén classification system, the intestinal type of adenocarcinoma was found to be the predominant subtype [14].
China has historically experienced a low rate of initial analysis for gastric tumor, with the majority of patients being diagnosed at advanced stages [15]. However, there have been efforts to improve early detection in recent years. During the period of 2016 to the year 2017, the prevalence of phase I gastric tumor diagnosis in China had been recorded at 15.5%, with urban areas showing higher rates in comparison to rural regions [14]. Notably, at a national level, the amount of cases identified with phase I GC in China had been reported to be 29.3%, demonstrating a significantly higher figure when compared to both the average proportion observed in our study and the overall Chinese average [16].
In contrast, Japan demonstrated higher rates of early-stage GC diagnosis in the 1990s, with about 50% of indivduals being diagnosed at an early stage [17]. The low proportion of early-stage gastric cancer diagnoses in China is a significant contributing factor to the lower survival rates among gastric cancer patients. Therefore, it is crucial to promote and encourage the adoption of endoscopic screening for GC in China to improve the early diagnosis rate [18]. In the early 20th century, around two-thirds of GC were primarily located in the antrum and prepyloric area,while only 10% were found in the cardia or the esophagogastric junction [19,20]
The IHC assay conducted in the present study revealed a strong correlation between CD40 and GC, independent of the patient's age or sex. The significant disparity in serum sCD40L levels between GC patients and controls led us to propose a hypothesis that suggests serum sCD40L might facilitate cancer development by suppressing immune activation. Previous research has provided evidence that CD40 and CD40L have a role in modulating anti-growth resistance by prompting the proliferation, initiation, development, and pvariation of different resistant cells [21]. However, the specific instrument that CD40- CD40L normalizes resistant jobs remains not completely understood. Several information suggests that the CD40-CD40L might delay the growing of tumor cells by inducing apoptosis and/or cell cycle arrest [22,22]. Moreover, scientific studies have indicated that the manifestation of CD40 or its binding agent on the exteriors of malignant cells triggers elevated quantities of Intercellular Adhesion Compound 1 (ICAM-1), augmentation of Fas manifestation, and increased susceptibility of tumor cells to Fas-induced programmed cell death [24]. In terms of the immediate influence on tumors, the growth necrosis aspect receptor-associated aspect (TRAF) is acknowledged as a pivotal intermediary molecule accountable for relaying CD40 signals to enable diverse physiological processes [25].
In a study conducted by Li et al. [26], it was observed that CD40 signaling plays a role in inhibiting apoptosis and promoting the proliferation of infected cells. These findings suggest that targeting the CD40 signaling pathway through potential therapeutic interventions could potentially help in controlling tumor cell proliferation in GC. Moreover, these revelations hold the potential to unlock the secrets of heightened biomarkers for formidable variants of gastric carcinoma [23,27].
CD40, belonging to the TNF superfamily, exhibits varying expression levels across different cell types, including epithelial cells, hematopoietic progenitor cells, and antigen-presenting cells. The CD40-CD40L costimulatory pathway plays a crucial role in facilitating humoral responses and the generation of cytokines like IL-10 and IL-12, which impact the functionality of T lymphocytes in their anti-tumor activities [28,29]. Recent studies conducted on mice have revealed that CD40 is not only essential for immune suppression mediated by myeloid-derived suppressor cells (MDSCs) but also for the expansion of tumorspecific regulatory T cells (Tregs). Notably, in an advanced tumor model, the inhibition of regulatory T cell (Treg) development and the enhanced efficacy of established immunomodulatory therapy were observed upon obstructive the collaboration among CD40L and CD40 using anti-CD40 antibodies [30]. Beyond its role in immune regulation, the binding of CD40-CD40L has a direct impact on tumor cell growth or apoptosis, as many tumor cells express CD40. The outcome is contingent upon the intensity of CD40L signaling, where advanced gesturing induces growth cell expiry, while minor gesturing encourages tumor evolution [31]. Indirectly, CD40L contributes to tumor growth through its involvement in angiogenesis, primarily mediated by factors like VEGF, TGF-β, and other chemokines. Studies in mice have demonstrated that CD40-CD40L stimulates VEGF production in endothelial cells and activates platelets, thereby promoting angiogenesis [30,30].
The ground breaking investigation [33] illuminates an intriguing nexus between the expression of a captivating CD40 distorted and the phases of gastric tumor, in addition to the tantalizing realm of forecast. Such a revelation beckons the tantalizing prospect of harnessing specific CD40 mutant antibodies to orchestrate a symphony of apoptosis in the besieged realm of gastric cancer cells. This awe-inspiring discovery kindles the flames of contemplation, suggesting that the enigmatic CD40 mutant could emerge as a beacon of hope, a novel target for the crusade of tumor immunotherapy and the art of immune intervention.
Enter the enigmatic CD40 mutant (CAC→CAA, 78His→78Gln, NCBI Assay ID: ss23134804, Reference SNP ID: rs17177493), a marvelously altered rendition of CD40, discovered gracing the surface of the illustrious U266 cell line and freshly isolated tumor cells. Nestled within its molecular tapestry, a singular mutation resides, nestling within a region of utmost importance, where the delicate embrace with CD40L transpires [34,35]. This intrepid CD40 mutant embarks on a transformative odyssey, traversing the realms of the CD40 signalosome, weaving its influence into the symphony of CD40 signal transduction. It modifies a partial epitope of its predecessor, endowing it with a distinct affinity, an uncanny ability to bind with antibodies honed for the wild-type CD40 [35].
Finally, in our study, we did not observe a clear effect of age or gender on the studied indicators, including CD40. We were unable to find a definitive explanation for this observation, suggesting the need for a larger-scale study involving more samples to determine whether age and sex play a role in controlling these immune indicators or cancer stem cell markers.
Conclusion
Immunohistochemistry assay determine that CD40 are highly expressed in gastric cancer cells. Serologically, in post or pre surgery, the level of CD40 evaluated in males compared to females without statistical differences (p>0.05). So, we found the highest concentration of CD40 after and before surgery in advanced metastatic cancer cases (stage IV) therefore, CD40 may be a therapeutic target for gastric cancer.
References
- Eliopoulos AG, Davies C, Knox PG. CD40 induces apoptosis in carcinoma cells through activation of cytotoxic ligands of the tumor necrosis factor superfamily. Mol Cell Biol. 2000;20:5503-15.
[Google Scholar] [ Crossref]
- Bates KM, Vathiotis I, MacNeil T. Spatial characterization and quantification of CD40 expression across cancer types. BMC Cancer. 2023;23:220.
[Google Scholar] [ Crossref]
- Ottaiano A, Pisano C, De Chiara A. CD40 activation as potential tool in malignant neoplasms. Tumori. 2002;88:361-6.[ Google Scholar] [ Crossref]
- Sadeghlar F, Vogt A, Mohr RU.Induction of cytotoxic effector cells towards cholangiocellular, pancreatic, and colorectal tumor cells by activation of the immune checkpoint CD40/CD40L on dendritic cells. Cancer Immunol Immunother. 2021;70:1451-1464.
[Google Scholar] [ Crossref]
- Tang T, Cheng X, Truong B. Molecular basis and therapeutic implications of CD40/CD40L immune checkpoint. Pharmacol Ther. 2021;219:107709.
[Google Scholar] [ Crossref]
- Li R, Chen WC, Pang XQ. Expression of CD40 and CD40L in gastric cancer tissue and its clinical significance. Int J Mol Sci. 2009;10:3900-3917.
[Google Scholar] [ Crossref]
- Wu YG, Wang L, He XZ. Expression of CD40 and growth-inhibitory activity of CD40 ligand in colon cancer ex vivo. Cell Immunol. 2008;253:102-109.
- Liu M, Liu L, Song Y. Targeting macrophages: a novel treatment strategy in solid tumors. J Transl Med. 2022;20:586.
[Google Scholar] [ Crossref]
- Portillo JA, Feliciano LM, Okenka G. CD40 and tumour necrosis factor-α co-operate to up-regulate inducible nitric oxide synthase expression in macrophages. Immunology. 2012;135:140-50.
[Google Scholar] [ Crossref]
- Djureinovic D, Wang M, Kluger HM. Agonistic CD40 Antibodies in Cancer Treatment. Cancers (Basel). 2021;13:1302.
- Taniguchi K, Ota M, Yamada T. Staging of gastric cancer with the Clinical Stage Prediction score. World J Surg Oncol. 2019;17:47.
- Zeng H, Ran X, An L. HBCR Working Group. Disparities in stage at diagnosis for five common cancers in China: a multicentre, hospital-based, observational study. Lancet Public Health. 2021;6:e877-e887.[ Google Scholar] [ Crossref]
- Ito T, Matoba R, Maekawa H. Detection of gene mutations in gastric cancer tissues using a commercial sequencing panel. Mol Clin Oncol. 2019;11:455-460.[ Google Scholar] [ Crossref]
- Attia S, Atwan N, Arafa M, Shahin RA. Expression of CD133 as a cancer stem cell marker in invasive gastric carcinoma. Pathologica. 2019;111:18-23.
[Google Scholar] [ Crossref]
- Li Q, Cai G, Li D. Better long-term survival in young patients with non-metastatic colorectal cancer after surgery, an analysis of 69,835 patients in SEER database. PLoS One. 2014;9:e93756.
[Google Scholar ] [Crossref]
- Kang W, Zeng H, Xiong J. Survival of patients with gastric cancer surgically treated at the National Cancer Center of China from 2011 to 2018 according to stage at diagnosis. J. Natl. Cancer Cent.,2022; 2: 132-138.[ Google Scholar]
[Crossref]
- Katai H, Ishikawa T, Akazawa K. Registration Committee of the Japanese Gastric Cancer Association. Five-year survival analysis of surgically resected gastric cancer cases in Japan: a retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001-2007). Gastric Cancer. 2018;21:144-154.
[Google Scholar] [ Crossref]
- Chen XZ, Zhang WH, Hu JK. A difficulty in improving population survival outcome of gastric cancer in mainland China: low proportion of early diseases. Med Oncol. 2014;31:315.
[Google Scholar] [ Crossref]
- Movahedi M, Afsharfard A, Moradi A. Survival rate of gastric cancer In Iran. J Res Med Sci. 2009;14:367-73.
[Google Scholar] [ Crossref]
- Abdi-Rad A, Ghaderi-Sohi S, Nadimi-Barfroosh H, Emami S. Trend in incidence of gastric adenocarcinoma by tumor location From 1969-2004: a study in one referral center in Iran. Diagn Pathol. 2006;1:5.
[Google Scholar] [ Crossref]
- Yanagimoto H, Takai S, Satoi S. Impaired function of circulating dendritic cells in patients with pancreatic cancer. Clin Immunol. 2005;114: 52-60.
[Google Scholar] [ Crossref]
- Quezada SA, Jarvinen LZ, Lind EF and Noelle RJ: CD40/CD154 Interactions at the interface of tolerance and Immunity. Annu Rev Immunol 22: 307-328, 2004.
[Google Scholar] [ Crossref]
- Scott K, Manunta M, Germain C. Qualitatively distinct Patterns of cytokines are released by human dendritic cells in Response to different pathogens. Immunology 116: 245-254, 2005.
[Google Scholar ] [Crossref]
- Schwulst SJ, Grayson MH, DiPasco PJ.Agonistic Monoclonal antibody against CD40 receptor decreases lymphocyte Apoptosis and improves survival in sepsis. J Immunol. 2006; 177: 557-565.
[Google Scholar ] [Crossref]
- Zirlik A, Bavendiek U, Libby P. TRAF-1, -2, -3, -5, and -6 Are induced in atherosclerotic plaques and differentially mediate Proinflammatory functions of CD40L in endothelial cells. Arterioscler Thromb Vasc Biol 27: 1101-1107.
[Google Scholar] [ Crossref]
- Li R, Chen WC, Pang XQ, Hua C, Li L, Zhang XG. Expression of CD40 and CD40L in gastric cancer tissue and its Clinical significance. Int J Mol Sci. 2009;10:3900-3917.
[Google Scholar] [ Crossref]
- Gallagher NJ, Eliopoulos AG, Agathangelo A.CD40 activation in epithelial ovarian Carcinoma Cells modulates growth, apoptosis, and cytokine Secretion. Mol Pathol 55: 110-120, 2002.
[Google Scholar ] [Crossref]
- Aggarwal BB. Signaling pathways of the TNF superfamily: A double-edged sword., Nat Rev Immunol, 2003; 3: 745-756.
[Google Scholar ] [Crossref]
- Elgueta R, Benson MJ, de Vries VC. Molecular mechanism and function of CD40/CD40L Engagement in the immune system., Immunol Rev, 2009; 229 :152-172.[ Google Scholar] [ Crossref]
- Bergmann S, Pandolfi PP. Giving blood: a new role for CD40 In tumorigenesis., J Exp Med, 2006; 203 : 2409-2412.
[Google Scholar] [ Crossref]
- Murugaiyan G, Martin S, Saha B. CD40-induced Countercurrent conduits for tumor escape or elimination?, Trends Immunol, 2007; 28: 467-473.
[Google Scholar] [ Crossref]
- Melter M, Reinders ME, Sho M. Ligation of CD40 Induces the expression of vascular endothelial growth factor by Endothelial cells and monocytes and promotes angiogenesis in vivo., Blood, 2000; 96: 3801-3808.
[Google Scholar] [ Crossref]
- Zhao, WQ., Li, XD., Shi, HB. CD40 mutant expression and its clinical significance to prognosis in gastric cancer patients. World J Surg Onc 12, 2014; 167.
[Google Scholar] [ Crossref]
- Hess S, Engelmann H: A novel function of CD40: induction Of cell death in transformed cells. J Exp Med. 1996, 183: 159-167.[ Google Scholar] [ Crossref]
- Qi CJ, Zheng L, Ma HB. A novel mutation in CD40 and its functional Characterization. Hum Mutat. 2009, 30: 985-994.[ Google Scholar] [ Crossref]

