Case Report - Onkologia i Radioterapia ( 2022) Volume 0, Issue 0
Liver transplantation for primary intrahepatic adenosquamous carcinoma using a marginal graft
Peng Chen1, Di Ma2, Ruokun Li3, Xiaoqun Yang4, Hui Tong1, Debin Qi1 and Tao Li1*2Department of Hepatobiliary Surgery, Shanghai Jiao Tong University School of Medicine, Shanghai, China
3Department of Radiology, Shanghai Jiao Tong University School of Medicine, Shanghai, China
4Department of Pathology, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Tao Li, Department of Surgery, Shanghai Jiao Tong University School of Medicine, ADDRESS: Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China Postcode: 200025, China, Email: transplant@126.com
Received: 19-May-2022, Manuscript No. OAR-22-64213; , Pre QC No. OAR-22-64213(PQ); Editor assigned: 23-May-2022, Pre QC No. OAR-22-64213(PQ); Reviewed: 06-Jun-2022, QC No. OAR-22-64213; Revised: 13-Jun-2022, Manuscript No. OAR-22-64213; Published: 20-Jun-2022, DOI: 10.4172/1896-8961.16.S1.003
Abstract
Background: Primary Intrahepatic Adenosquamous Carcinoma (iASC) is a very rare subtype of Cholangiocarcinoma (CCA), with a worse prognosis than adenocarcinoma. Hepatectomy is still the first choice for iASC, and Liver Transplantation (LT) for iASC has not been previously reported.
Case presentation: The young male patient with an unresectable primary iASC underwent a LT using a marginal fatty liver graft. The overall survival of this patient was 16 months, with a recurrence-free survival of 8 months. The patient had a good quality of life with normal liver function until 3 months before death.
Conclusion: This is the first case report of LT as treatment of unresectable iASC using a marginal graft, by which our patient gained a longer survival. Although the recipient may benefit from LT, LT for iASC should proceed with caution owing to its more aggressive features.
Keywords
Case report, cancer/malignancy/neoplasia, primary adenosquamous carcinoma, cholangiocarcinoma, liver transplantation/hepatology
Introduction
Esophageal Primary Intrahepatic Adenosquamous Carcinoma (IASC) is defined by the coexistence of both squamous and glandular elements in the same lesion histologically. IASC is clinically more aggressive with a worse prognosis, which accounts for only 2% to 3% of Cholangiocarcinoma (CCA), and a majority of patients died within 1 year [1]. We herein describe a patient with primary IASC who underwent orthotopic Liver Transplantation (LT). To our knowledge, this is the first reported case for IASC treated by LT.
Case Representation
A 28-year-old man was admitted to our hospital complaining of a 10-day history of fatigue, upper abdominal discomfort, and a 5-day history of jaundice. He admitted a history of ulcerative colitis for 2 years and congenital cyst of biliary track. No abnormalities but jaundice was found on systemic examinations. The relevant laboratory results were as follows: WBC 13.11 × 109/L, Alanine Aminotransferase (ALT) 299 IU/L, Total Bilirubin (TBIL) 586.0 μmol/L, albumin 26 g/L, carbohydrate antigen (CA) 19-9 453.5 U/mL, CA 125 21.60 U/mL, Carcinoembryonic Antigen (CEA) 1.73 ng/mL, Alpha-Fetoprotein (AFP) 4.69 ng/mL, Hepatitis B and hepatitis C were both negative. Abdominal enhanced Magnetic Resonance Imaging (MRI) and Magnetic Resonance Cholangiopancreatography (MRCP) revealed an irregular mass (approximately 48 mm × 44 mm in its dimensions) mainly located at segment VIII (S8) of the liver involving the hepatic hilum with mild delayed enhancement, a small metastatic lesion at segment VII (S7), and several small sporadic cysts in the liver (Figure 1A-1C). Positron Emission Tomography/Computed Tomography (PET/CT) clearly demonstrated hotspots (SUV max: 7.7 for the primary mass and 4.2 for the metastatic lesion) at the same locations as the tumors detected by MRI, but no hot spots for lymph nodes and distant metastases were observed (Figure 1D). Taking together, the clinical preoperative diagnosis of intrahepatic perihilar CCA with S7 metastasis was made and it was unresectable.
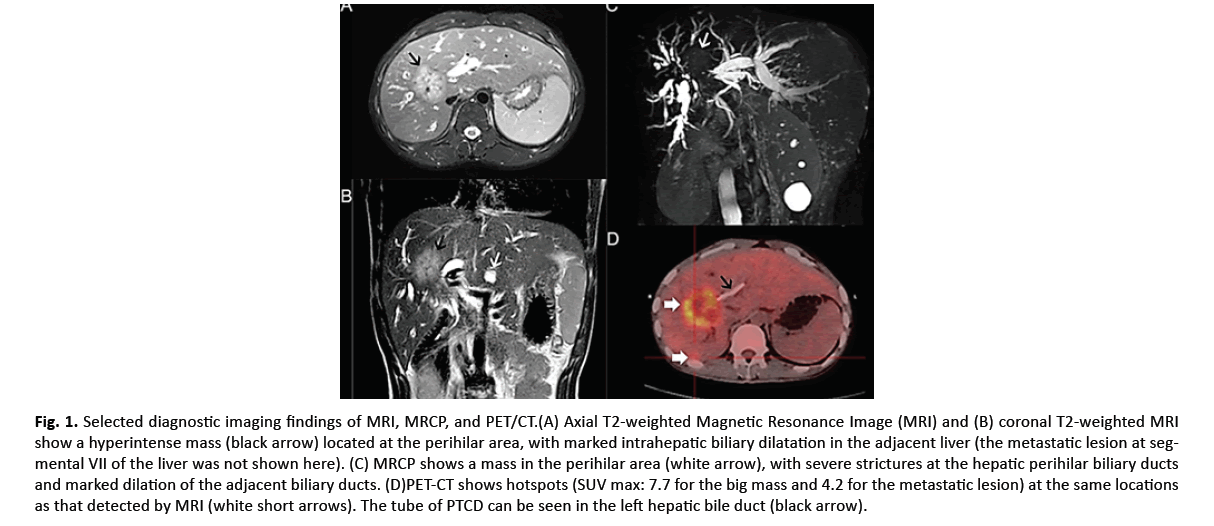
Figure 1: Selected diagnostic imaging findings of MRI, MRCP, and PET/CT.(A) Axial T2-weighted Magnetic Resonance Image (MRI) and (B) coronal T2-weighted MRI show a hyperintense mass (black arrow) located at the perihilar area, with marked intrahepatic biliary dilatation in the adjacent liver (the metastatic lesion at segmental VII of the liver was not shown here). (C) MRCP shows a mass in the perihilar area (white arrow), with severe strictures at the hepatic perihilar biliary ducts and marked dilation of the adjacent biliary ducts. (D)PET-CT shows hotspots (SUV max: 7.7 for the big mass and 4.2 for the metastatic lesion) at the same locations as that detected by MRI (white short arrows). The tube of PTCD can be seen in the left hepatic bile duct (black arrow).
After the ethical approval and consent were clearly and fully obtained from the Ruijin Hospital’s Ethical Committee, this patient was enrolled in the national waiting list of LT. A marginal fatty liver graft from a donor of brain death was allocated via the China Organ Transplant Response System two months after admission. After the Percutaneous Transhepatic Cholangiodrainage (PTCD), LT with hepatoduodenal ligament lymph node skeletal dissection was performed successfully. The postoperative initial immunosuppression consisted of tacrolimus and mycophenolate without hormone. Histopathologically, the lesions were consisted of malignant squamous cells and a glandular component, accompanied by invasion of the perihilar bile ducts and metastases of the lymph nodes (Figure 2A-2C). Immunohistochemical study showed p40 and p63 protein were strongly positive (Figure 3A and 3B). Meanwhile, Cytokeratin 7 (CK-7) and mucin 1 (MUC1) labelling adenocarcinoma were strongly positive where p40 and p63 were negative (Figure 3C and 3D).
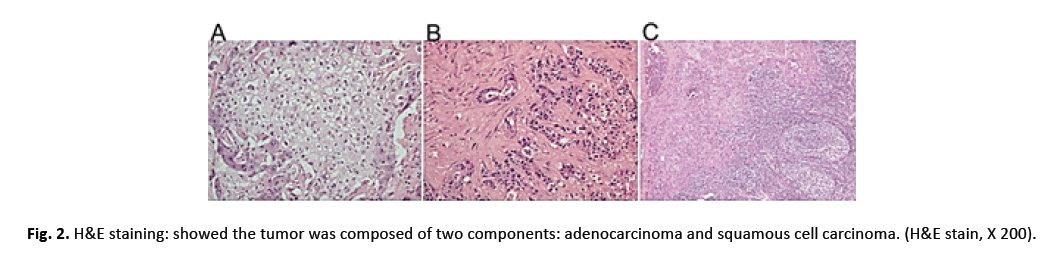
Figure 2: H&E staining: showed the tumor was composed of two components: adenocarcinoma and squamous cell carcinoma. (H&E stain, X 200).
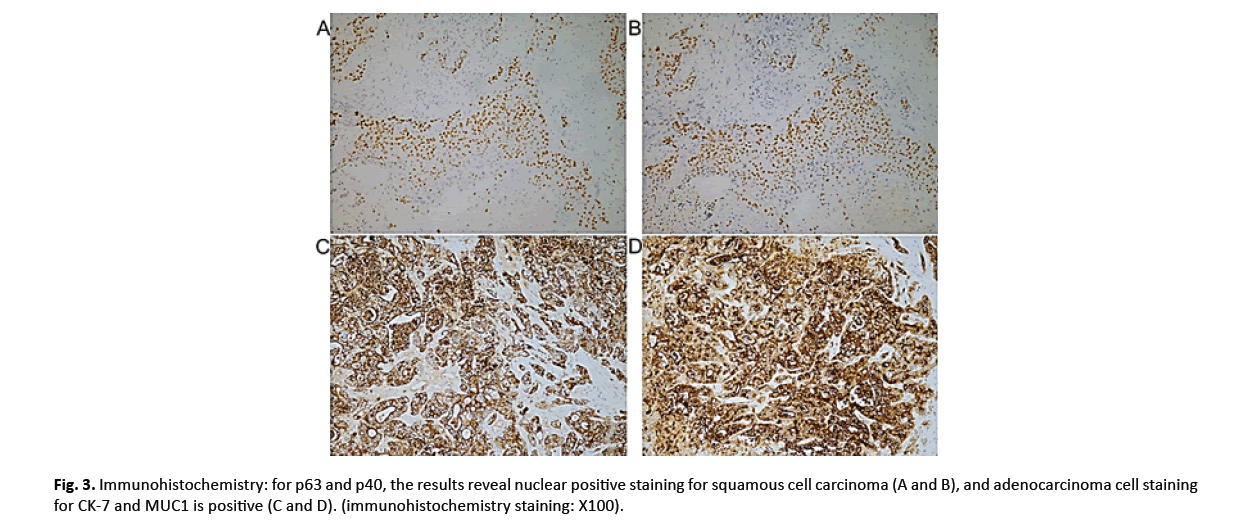
Figure 3: Immunohistochemistry: for p63 and p40, the results reveal nuclear positive staining for squamous cell carcinoma (A and B), and adenocarcinoma cell staining for CK-7 and MUC1 is positive (C and D). (immunohistochemistry staining: X100).
The patient recovered well and was discharged 14 days postoperatively. On postoperative four weeks, the immunosuppressive regimen was converted to sirolimus-based immunosuppressive therapy. The patient received the first cycle of capecitabine chemotherapy 2 months after surgery and totally 4 cycles. Despite of intensive treatment strategies, this patient showed an increase in CA19-9 eight months after surgery without any discomforts (Figure 4). Chest Computed Tomography (CT) revealed tumor metastases of the thoracic vertebras. The patient then received a reduction of immunosuppressive drugs, systemic capecitabine chemotherapy, and infusion of zoledronic acid which has a significant clinical benefit in bone metastases from multiple tumor types [2]. The gene mutations related to CCA in peripheral circulating tumor DNA (ctDNA) were detected by next-generation sequencing in order to identify if there were effective targeted drugs. Unfortunately, the results showed no mutations of typical genes, like EGFR, IDH1, IDH2 [3].
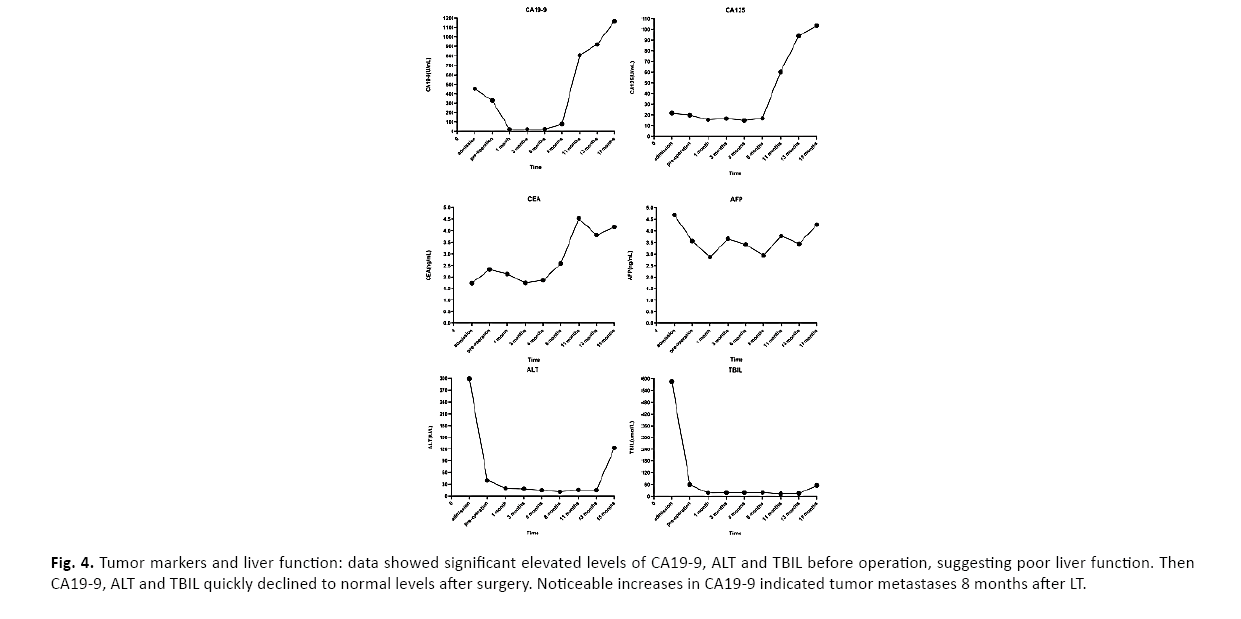
Figure 4: Tumor markers and liver function: data showed significant elevated levels of CA19-9, ALT and TBIL before operation, suggesting poor liver function. Then CA19-9, ALT and TBIL quickly declined to normal levels after surgery. Noticeable increases in CA19-9 indicated tumor metastases 8 months after LT.
This patient with IASC died from multiple metastases of tumor eventually. The overall survival and recurrence-free survival are 16 months and 8 months, correspondi ngly (Figure 5). Of note, the patient had a good quality of life with normal liver function until 3 months before death.
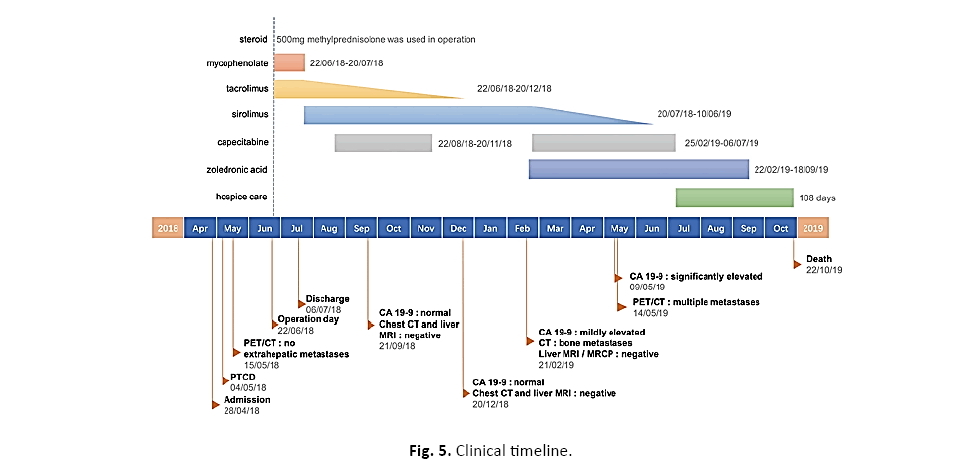
Figure 5: Clinical timeline.
Results and Discussions
Primary iASC is a very rare histological type of CCA, consisting of adenocarcinoma and squamous cell carcinoma components in the same tumor [4]. Since 1971, when iASC was first reported by Pianzola and Durt [5], including our case, about 90 cases have been published. Due to its rarity, the clinicopathological features and biological behaviors have been incompletely elucidated. The main initial symptoms of iASC are fever, weight loss and epigastric pain [6], which are not characteristic. Jaundice may occur in biliary obstruction due to hilar bile duct invasion or biliary lithiasis.
The common tumor markers for iASC are CA 19-9 and CEA. However, CA 19-9 can also be very high in obstructive jaundice [7]. In this case, tumor was sensitive to CA 19-9, and an increase level of CA 19-9 eventually suggested tumor recurrence.
Enhanced CT and MRI with MRCP are first choice to assess the tumor and vascular involvement. ERCP can place biliary stent to drain bile in patients with biliary obstruction while invasiveness and low biopsy positive rate limit its clinical use. PET/CT can be a reliable tool for detection of CCA and metastatic lymph nodes with an accuracy of 80% approximately [8]. Thus, laparoscopical lymph node biopsy closer to the time of transplantation are recommended in order to rule out nodal metastases regardless of imaging appearance.
Pathological features of iASC need to be elucidated. Cell keratinization, intercellular bridges, keratin pearl formation and/or dyskeratosis are considered to be squamous cell carcinoma components while various-size of gland formations and intracellular and/ or intraluminal mucin give evidence to glandular differentiation. Moreover, Immunohistochemistry (IHC) is crucial in diagnosis, like p40/63 and CK5/6 indicating squamous differentiation [9], while CK-7, MUC1 are always positive in adenocarcinoma [10]. In accordance with the IHC findings, we successfully made a pathological diagnosis of IASC.
The pathogenesis of IASC is not well understood. Chronic inflammation associated with bile duct infection or biliary lithiasis and various congenital cysts of the biliary tract, are two main reasons of metaplastic changes in the biliary epithelium, ultimately ending with malignant neoplasia [11]. In our case, persistent irritations from stones and congenital cysts might contribute to the development of IASC. Recent study show inflammatory bowel disease is also a risk factor for CCA [12], the chronic colitis in our patient may lead to IASC. Some authors [13], advocated the squamous cell carcinoma component might arise from squamous metaplasia of adenocarcinoma cells in the same lesion [14], this hypothesis has been confirmed by an experimental animal model, showing squamous cell carcinoma is a result of histopathology alteration from adenocarcinoma as a transitional form [15]. In this case, all tumors contained squamous cell carcinoma and adenocarcinoma without normal bile duct epithelium as hypothesis.
The first-line treatment of choice for IASC is hepatectomy. Unfortunately, most patients with IASC are diagnosed with either unresectable locally-advanced or metastatic disease at presentation. In such unresectable cases, LT is theoretically an attractive option, as it offers the maximum resection margin regardless of the status of the liver function and the volume of residual liver. The prognosis of IASC has been reported to be extremely poor, even with resection. Kobayashi analyzed that the mean survival of 34 patients with IASC was only 8.74 months [6].
Historically, early experience with LT for intrahepatic CCA (iCCA) was discouraging with essentially poor overall survival rates and increased recurrence [16]. Due to the scarce resources of grafts, the main concern about offering a liver donor to these patients is the influence on other patients on the waiting list. Therefore, LT to treat patients with CCA is confined to those with an unresectable hilar CCA especially meeting the “Mayo clinic protocol” or with “very early” (single tumor <2 cm) iCCA in most liver transplant centers [17]. Obviously, the patient in our case was beyond these rigorous selection standards for CCA. Considering this young patient’s age and no extrahepatic metastases, LT using a marginal fatty liver donor which was rejected in patients on waiting list was eventually approved by the organ transplantation ethic committee of our hospital to prolong his life maximumly. Due to the different organ allocation policies in various countries, in some with relatively sufficient donors, the use of marginal liver grafts as exploratory therapy may give some patients with advanced liver tumors a chance to cure.
In view of the patient’s poor liver function, preoperative adjuvant therapy was not performed, instead he received oral capecitabine chemotherapy 2 months after LT. Calcineurin Inhibitors (CNIs) combined corticosteroid are regarded as the first line immunosuppressive therapy for LT, but some clinical studies have revealed a CNIs dose-dependent increase in the post-transplant risk of Hepatocellular Carcinoma (HCC) relapse [18]. Corticosteroid-free immune-suppression which is performed as a routine immunosuppressive regimen for HCC in our center has been reported to be safe for patients with HCC and showed an advantage in reducing the recurrence of HCC [19]. Mammalian target of rapamycin inhibitors, like sirolimus and everolimus, have shown an antineoplastic effect in vitro and clinical studies. In a meta-analysis, sirolimus was associated with a lower incidence of HCC recurrence after LT, compared with CNIs [20]. Thus, in our case, the patient received an initial steroid-free tacrolimus-based immunosuppressive regimen after LT and was converted tacrolimus to rapamycin as the central immunosuppressive drug 4 weeks after operation. The incision had healed 4 weeks after operation, so the switch to rapamycin would not add the incidence of its side effects that have been reported previously, like delayed wound healing, incisional hernias and arterial thrombosis [21,22].
Conclusion
In spite of the above intensive prevention measures in our case, the metastases were found about 8 months after surgery and progressed rapidly. The patient was given minimal immunosuppressive drugs, continued systemic capecitabine chemotherapy and palliative treatment without targeted therapy after metastases. Molecularly targeted therapy based on the key signaling pathways and mutated genes may be an option for unresectable or metastatic CCA patients, either alone or in combination with chemotherapy and immunotherapy. CtDNA allows for the evaluation of patient-specific tumoral genetic and epigenetic alterations, and the mutations found in ctDNA which are highly representative in iCCA may bring a large number of potential therapeutic targets [3]. Unfortunately, the most frequently mutated genes in CCA were not detected in peripheral ctDNA, suggesting IASC might have a very different genomic profile from conventional CCA. The genotyping needs further studies to make a better understanding on this rare tumor. The overall survival of this patient was 16 months, which exceeded that of the most patients with IASC undergoing liver resection in previous literatures.
Undoubtedly, the rarity of IASC sets an obstacle for improving the diagnosis and treatment of such disease, more clinical data should be collected in the future. In this case, the use of marginal liver grafts in LT as exploratory therapy may give patients with advanced IASC a chance to cure, which significantly prolong the survival of IASC than average. However, owing to the aggressive features of IASC and the lack of effective adjuvant drugs at present, LT for IASC should proceed with caution.
Acknowledgments
The authors thank Haitao Wen for his kind linguistic revision.
Funding
The authors declare that none type of funding was received for this work.
Availability of Data and Materials
The clinical data used or analyzed in this case report are available from the corresponding author on reasonable request.
Author's Contributions
Peng Chen and Di Ma are co-first authors. All authors contributed to the writing and revision of the article. Peng C and Di M contributed equally to this work. Peng C, Ma D and Tao L contributed to the conception of study. Ruokun L, Xiaoqun Y, Tong H and Debin Q contributed to data collection and performance of the research.
Ethics Approval and Consent to Participate
The ethical approval and consent were clearly and fully obtained from the Ethical Committee of Ruijin Hospital, the Affiliated Hospital of Shanghai Jiao Tong University School of Medicine. Patient was provided with written informed consent based on the Declaration of Helsinki.
Cosent for Publication
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Competing Interests
The authors declare that they have no competing interests.
References
- Nakajima T, Kondo Y. A clinicopathologic study of intrahepatic cholangiocarcinoma containing a component of squamous cell carcinoma. Cancer. 1990;65:1401-1404.
- Honda Y, Takahashi S, Zhang Y, et al. Effects of bisphosphonate zoledronic acid in hepatocellular carcinoma, depending on mevalonate pathway. J Gastroenterol Hepatol. 2015;30:619-627.
- Ettrich TJ, Schwerdel D, Dolnik A, et al. Genotyping of circulating tumor DNA in cholangiocarcinoma reveals diagnostic and prognostic information. Sci Rep. 2019;9: 13261.
- Takahashi H, Hayakawa H, Tanaka M, et al. Primary adenosquamous carcinoma of liver resected by right trisegmentectomy: report of a case and review of the literature. J Gastroenterol. 1997;32:843-847.
- Pianzola LE, Drut R. Mucoepidermoid carcinoma of the liver. Am J Clin Pathol. 1971;56:758-761.
- Kobayashi M, Okabayashi T, Okamoto K, et al. A clinicopathologic study of primary adenosquamous carcinoma of the liver. J Clin Gastroenterol. 2005;39:544-548.
- Giron F, Alcantar D. Looks can be deceiving: a case report on the clinical value of CA 19-9 in obstructive jaundice. Cureus. 2020;12:e6637.
- Li X, Zhang Y, Zhang Y. 18F-FDG PET/CT may be a suitable method for preoperative diagnosis and evaluation of Chinese older patients with hilar cholangiocarcinoma. BMC Geriatr. 2018;18:150.
- Nobre AR, Albergaria A, Schmitt F. p40: A p63 isoform useful for lung cancer diagnosis: A review of the physiological and pathological role of p63. Acta Cytol. 2013;57:1-8.
- Rao N. Adenosquamous carcinoma. Semin Diagn Pathol. 2014;31:271-277.
- Lu BJ, Cao XD, Yuan N, et al. Concomitant adenosquamous carcinoma and cystadenocarcinoma of the extrahepatic bile duct: A case report. World J Clin Cases. 2019;7:215-220.
- Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54:173-184.
- Barr RJ, Hancock DE. Adenosqu7amous carcinoma of the liver. Gastroenterology. 1975;69:1326-1330.
- Yamana I, Kawamoto S, Nagao S, et al. Squamous cell carcinoma of the hilar bile duct. Case Rep Gastroenterol. 2011; 5: 463-470.
- Iemura A, Yano H, Mizoguchi A, et al. A cholangiocellular carcinoma nude mouse strain showing histologic alteration from adenocarcinoma to squamous cell carcinoma. Cancer. 1992; 70:415-422.
- Lunsford KE, Javle M, Heyne K, et al. Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: a prospective case-series. Lanc Gastroe Hepa. 2018;3:337-348.
- Goldaracena N, Gorgen A, Sapisochin G. Current status of liver transplantation for cholangiocarcinoma. Liver Transpl. 2018;24:294-303.
- Hojo M, Morimoto T, Maluccio M, et al. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature. 1999;397:530-534.
- Wei Q, Xu X, Wang C, et al. Efficacy and safety of a steroid-free immunosuppressive regimen after liver transplantation for hepatocellular carcinoma. Gut Liver 2016;10:604-610.
- Grigg SE, Sarri GL, Gow PJ, et al. Systematic review with meta-analysis: sirolimus-or everolimus-based immunosuppression following liver transplantation for hepatocellular carcinoma. Aliment Pharmacol Ther. 2019; 49:1260-1273.
- Pridöhl O, Heinemann K, Hartwig T, et al. Low-dose immunosuppression with FK 506 and sirolimus after liver transplantation: 1-year results. Transplant Proc. 2001;33: 3229-3231.
- DeLeon TT, Ahn DH, Bogenberger JM, et al. Novel targeted therapy strategies for biliary tract cancers and hepatocellular carcinoma. Future Oncol. 2018;14:553-566.

