Research Article - Onkologia i Radioterapia ( 2021) Volume 15, Issue 3
Magnetic resonance imaging for soft tissue tumors in comparison to histopathology
Sanaa Jawad Kadhim1, Tara Farooq Kareem2 and Sahar Ahmed Mahdi32Department of Radiology, Oncology Teaching Hospital, Baghdad Medical City Complex, Ministry of Health/Environment, Baghdad, Iraq
3Department of Radiographic Techniques, Al-Turath University, Baghdad, Iraq
Received: 10-Jan-2021 Accepted: 10-Mar-2021 Published: 17-Mar-2021
Abstract
Background: Soft tissue masses are frequently referred for imaging assessment. Magnetic Resonance Imaging (MRI) is the technique of choice for characterization and local staging of a soft tissue masses.
Objectives: The study aimed to assess the utility of MRI in differentiating benign from malignant Soft Tissue Tumors (STTs).
Methods: A prospective study was conducted in the Radiology Department of the Oncology Teaching Hospital, Baghdad Medical City complex. A total of 37 patients were enrolled during the period from 12th July 2019 to 20th February 2020. The study sample consisted of 12 male and 25 female. MRI examination was performed utilizing the 1.5 Tesla Siemens system (Germany).
Results: The mean age of participants was 37.65 ± 16.59 years. The benign STTs were recorded in 15 (40.5%), while malignant tumors found in 22 (59.5%) of patients. The majority was on the left lower limb as 10 (27%). Those with tumor mass size below 5 cm were 8 (21.6%), whereas those above or equal to 5 cm were 29 (78.4%). The mean masses size was 10.2 ± 6.43 cm, with median size reached to 8.8 cm. The histopathological diagnosis of masses were achieved either by excisional biopsy in 28 (75.7%) or by true-cut biopsy in 9 (24.3%). The results of histopathology were: 16.2% hemangioma, 13.5% benign neurogenic tumor, 10.8% myxoid liposarcoma, 8.1% Malignant Peripheral Nerve Sheath Tumor (MPNST), 5.4% for each B- cell NHL, fibromatosis, pleomorphic sarcoma, and Ewing sarcoma, and 2.7% for each other’s pathology. The most common site for malignant tumor was in lower extremities, (p=0.031). The tumor masses distributions were statistically significant differences among different sites particularly in the lower limbs sites in malignant tumors (p=0.04). The relation between large tumors sizes and malignant pathology was statistically significant differences (p=0.042). The clear fluid non-enhancing component (that represents serous fluid) might be more evident in malignant rather than benign STTs with significant differences (P=0.028). In addition, the immediate enhancement of the tumor had strong association with malignant than benign tumors, (p=0.007).
Conclusions: The larger lesion sizes, the higher the probability of being malignancy. Hemangioma was the most common benign STTs diagnosed, while the most common malignant STTs were myxoid liposarcoma.
Keywords
soft tissue tumors, MRI, myxoid liposarcoma, hemangioma
Introduction
Soft tissue masses may be benign, malignant or non-neoplastic and all may present in a similar manner. Benign and nonneoplastic masses are more common, with benign lesions frequently said to occur approximately one hundred times more commonly than malignant [1].
MRI is the technique of choice for local staging of a soft tissue mass, especially if it is in a deep location where US may be less able to assess tumour extent and relations. It is sensitive, can be tissue-specific and allows assessment of most masses, no matter where they are located. Despite this, MRI is often not sufficiently tissue specific to allow confident identification of some deep, solid masses; distinction of myxoid from cystic masses can be difficult without contrast medium and small calcific foci may not be seen [2].
MR imaging is well suited for not only the diagnosis but also for the staging, preoperative planning, postsurgical evaluation, and post-therapy surveillance of soft tissue tumors [3].
A combination of T1- and T2-weighted images is the mainstay of MR imaging of soft tissue tumors. Fat-suppression techniques are widely adopted to enhance the dynamic ranges and sensitivity of fast spin echo T2-weighted images and gadolinium-enhanced T1-weighted images. The administration of intravenous gadolinium chelates is used to distinguish cystic from solid components to identify viable and necrotic areas, to show the relative vascularity of tumors, and to delineate the true margin of tumors [4].
Although benign tumors tend to be well delineated and some malignant tumors have ill-defined margins, several studies have concluded that the margin of a soft tissue mass on MR imaging is of no statistical relevance in predicting malignancy [5]. In superficial soft tissue tumors, which are defined as masses located within the subcutaneous layer, the following various imaging features are known to be related to malignancy: lobulation, hemorrhage, necrosis, fascial edema, skin thickening, and skin contact. However, size was not found to be an important determining factor for malignancy, with a significant proportion of malignant superficial sarcomas measuring less than 5 cm in maximal diameter [6].
Patients and Methods
Study design
After approval by the College of Medicine/University of Baghdad, a prospective study was conducted in the Radiology Department of the Oncology Teaching Hospital, Baghdad Medical City complex.
Study setting
Total 37 patients with soft tissue mass were enrolled in the study during the period from 12th July 2019 to 20th February 2020. The study sample consisted of 12 male and 25 female, with their age, range from 13-70 years (mean=38.39 ± 16.59 years).
Data collection
Clinical data, including age, gender, tumor sites, maximal sizes, histopathology, anatomic location, and biopsy method (truecut biopsy or surgical excision) were gathered, and the results of tissue biopsies, based on review of medical records. All the studied patients went for MRI examination, which was done before any intervention.
Exclusion criteria
1. Any contraindication for MRI examination (pacemaker, cochlear implants, claustrophobia, pregnancy, previous allergy to gadolinium contrast).
2. Patients who refused to do the MRI examination.
3. Patients sent for the MRI re-evaluation of the mass after radiotherapy.
4. Patients with masses that showed no enhancement (as lipomas and a case of an intramuscular hydatid cyst).
5. A case of elastofibroma dorsi was excluded (as it has typical imaging features with no biopsy needed to prove the diagnosis).
6. Patients with no histopathological result (no feedback).
7. Pediatrics age groups.
8. Patients with soft tissue swelling after trauma.
MRI protocols
MRI examination was performed utilizing the 1.5 Tesla Siemens system (Magnetom Aera; Siemens Healthineers, Erlangen, Germany).
The protocol applied in our examined patients included: T1 (t1- fl2d) axial; T2 (t2- tse) axial; T2 fat suppression (t2-haste-fs) axial; T2 (t2-tse) coronal/or sagittal (according to the location of the mass); T1 fat suppression (t1-tse-fs) axial and coronal /or sagittal before and after injection of IV contrast (gadolinium) at a dose of 0.1 mmol/kg. In few cases, post contrast images were taken without fat suppression.
The post contrast images were taken in the early phase (45 sec-60 sec post injection) for all the cases, and additional post contrast images were taken in the delayed phase (5 minutes post injection) in cases that showed masses with very hyperintense signal in T2WI.
Ethical Considerations
Written informed consent was obtained from the patients for participating in this study. The study conforms to the 1995 Helsinki declaration and was approved by The Medical Ethical Committee of College of Medicine/Baghdad University.
Statistical Analysis
Statistical package for social science (SPSS statistics for windows, version 11.0, Chicago: SPSS, Inc.) software version 20 was used. Results were described in the form of frequencies and percentage distribution for qualitative data and (mean, SE of mean and standard deviation) calculation for quantitative data. Paired samples T test was used to estimated differences in quantitative variables. Pearson’s correlation test was used to detect the relationship between continuous variables as cross tabulation for comparison between values. A one-sided p value of 0.05 or less was considered statistically significant.
Results
The most commonly recorded age group was that between 21- 30 and 51-60 years as 8 (21.6%). The mean age was 37.65 ± 16.159 years with median equal to 38 years. Regarding gender, 12 (32.4%) were male, and 25 (67.6%) of patients were female, (Table 1).
Tab. 1. Patients demographic distribution of this study (n=37)
| Characteristics | No. | % | |
|---|---|---|---|
| Age (years) | Nov-20 | 7 | 18.9 |
| 21-30 | 8 | 21.6 | |
| 31-40 | 5 | 13.5 | |
| 41-50 | 7 | 18.9 | |
| 51-60 | 8 | 21.6 | |
| >60 | 2 | 5.4 | |
| Gender | M | 12 | 32.4 |
| F | 25 | 67.6 | |
Regarding the sites of STTs, the majority was on the left lower limb as 10 (27%). Those with tumor mass size below 5 cm were 8 (21.6%), whereas those above or equal to 5 cm were 29 (78.4%). The mean masses size was 10.2 ± 6.43 cm, with median size reached to 8.8 cm. The smallest tumor was 2.2 cm, while the largest size was 30 cm. Benign STTs were recorded in 15 (40.5%) patients, while malignant tumors were found in 22 (59.5%) of patients. Superficial lesions were figured in 9 (24.3%) of patients. Deep masses were found in 28 (75.7%) of patients, as shown in (Table 2).
| Variables | No. | % | |
|---|---|---|---|
| Sites | Anterior abdominal wall | 5 | 13.5 |
| Anterior chest wall | 1 | 2.7 | |
| Gluteal subcutaneous fat or muscle | 2 | 5.4 | |
| Infra-temporal muscle | 2 | 5.4 | |
| Left lower limb | 10 | 27 | |
| Scalp | 5 | 13.5 | |
| Left arm (intramuscular) | 2 | 5.4 | |
| Pelvic floor muscle | 1 | 2.7 | |
| Posterior to the calcaneoum | 1 | 2.7 | |
| Right arm | 1 | 2.7 | |
| Right lower limb | 6 | 16.2 | |
| Back (subcutaneous tissue) | 1 | 2.7 | |
| Sizes | <5 cm | 8 | 21.6 |
| ≥ 5 cm | 29 | 78.4 | |
| Types | Benign | 15 | 40.5 |
| Malignant | 22 | 59.5 | |
| Locations | Superficial | 9 | 24.3 |
| Deep | 28 | 75.7 | |
The histopathological diagnosis of masses were achieved either by excisional biopsy in 28 (75.7%) or by true-cut biopsy in 9 (24.3%). The results of histopathology were: 16.2% hemangioma, 13.5% benign neurogenic tumor (no myxoid component), 10.8% myxoid liposarcoma, 8.1% MPNST, 5.4% for each B- cell NHL, fibromatosis, pleomorphic sarcoma, and Ewing sarcoma, and 2.7% for each other’s pathology which listed in (Table 3).
Tab. 3. Histopathological findings of this study (n=37)
| Variables | No. | % | |
|---|---|---|---|
| Methods | True cut | 9 | 24.3 |
| Excisional | 28 | 75.7 | |
| B-cell NHL | 2 | 5.4 | |
| Benign myxoid neurofibroma | 1 | 2.7 | |
| Intramuscular myxoma | 1 | 2.7 | |
| Benign nerve sheath tumor(non myxoid type) | 5 | 13.5 | |
| Dermatofibrosarcoma protuberance | 1 | 2.7 | |
| Extra axial osteogenic sarcoma | 1 | 2.7 | |
| Extramedullary plasmacytoma | 1 | 2.7 | |
| Fibromatosis | 2 | 5.4 | |
| Hemangioma | 6 | 16.2 | |
| Tissue biopsy results | Lieomyosarcoma | 1 | 2.7 |
| Liposarcoma (non myxoid type) | 1 | 2.7 | |
| Malignant peripheral nerve sheath tumor | 3 | 8.1 | |
| Metastatic adenocarcinoma | 1 | 2.7 | |
| Myeloid sarcoma | 1 | 2.7 | |
| Myxoid liposarcoma | 4 | 10.8 | |
| Pleomorphic sarcoma | 2 | 5.4 | |
| Malignant fibrous histiocytoma | 1 | 2.7 | |
| Ewing sarcoma | 2 | 5.4 | |
| Fibrosarcoma | 1 | 2.7 | |
The enhancing component of all the 37 masses in our research (100%) showed isointense or hypointense signal in T1WI (as compared to the nearby muscles). Regarding the signal intensity of the enhancing component in T2WI, most of the masses (25/37) (67.6%) showed intermediately hyperintense signal in T2WI. One case (1/37) (2.7%), which was proved to be myeloid sarcoma showed hypointense signal in T2WI. The rest of the cases (11/37) (29.7%) the enhancing solid component showed very hyperintense signal in T2WI. The homogenous picture of enhancement presented in 15 (40.5%), while the heterogeneous enhancement was noted in 22 (59.5%). Immediate enhancement was seen in 32 (86.5%), whereas gradual enhancement occurred in 5 (13.5%), which were all suggested and proved by histopathology to be hemangiomas, as shown in (Table 4).
Tab. 4. Soft tissue masses details by MRI (n=37)
| Variables | No. | % | |
|---|---|---|---|
| Isointense or | |||
| T1W1 | hypointense | 37 | 100 |
| Hyperintense | 0 | 0 | |
| Hypointense | 1 | 2.7 | |
| Intermediately hyperintense | 25 | 67.6 | |
| T2W1 | Very hyperintense | 11 | 29.7 |
| Homogenous | 15 | 40.5 | |
| Enhancement | Heterogeneous | 22 | 59.5 |
| Fluid | 15 | 68.2 | |
| The non- enhancing component | Blood | 2 | 9.1 |
| Fat | 3 | 13.6 | |
| Fibrosis | 2 | 9.1 | |
| Immediate | 32 | 86.5 | |
| Enhancement | Gradual | 5 | 13.5 |
Regarding MRI sequences used, we could reach the definite diagnosis whether the mass is benign or malignant (as correlated with the biopsy result) depending on specific signs noted in (12/37): Benign neurogenic tumor (target sign noted) (2/12); Benign neurogenic tumor (due to the fat split sign) (1/12); Liposarcomas (due to the fat non enhancing component besides the enhancing solid component) (3/12); Hemangiomas (due to the gradual enhancement in post contrast study with the very hyper intense signal changes in T2WI) (4/12); Sarcoma (due to overlying skin invasion ) (2/12).
In the rest of the cases (24/37), the MRI couldn’t help reaching the definitive diagnosis or definite differentiation between benign and malignant masses given either a differential diagnosis in the report or a diagnosis that was discordant with the histopathological result (as in a case of Malignant peripheral nerve sheath tumor that was interpreted at the report as a benign plexiform neurofibroma due to multiple target signs but the histopathology showed malignant changes).
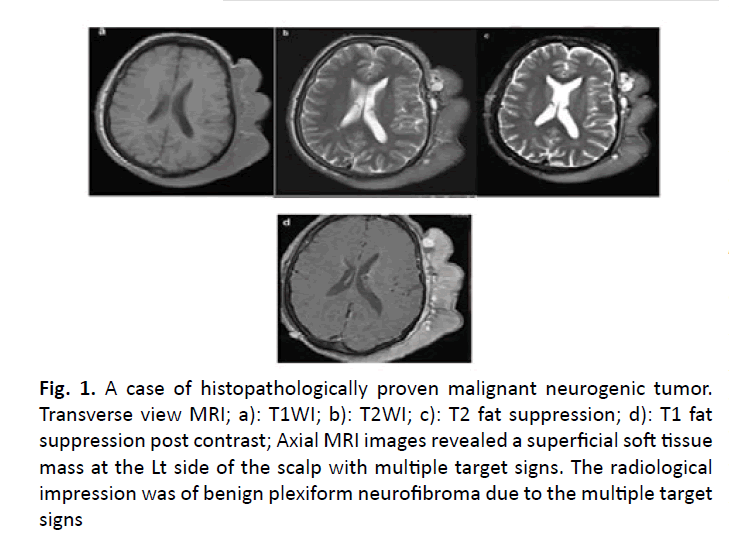
Figure 1: A case of histopathologically proven malignant neurogenic tumor. Transverse view MRI; a): T1WI; b): T2WI; c): T2 fat suppression; d): T1 fat suppression post contrast; Axial MRI images revealed a superficial soft tissue mass at the Lt side of the scalp with multiple target signs. The radiological impression was of benign plexiform neurofibroma due to the multiple target signs
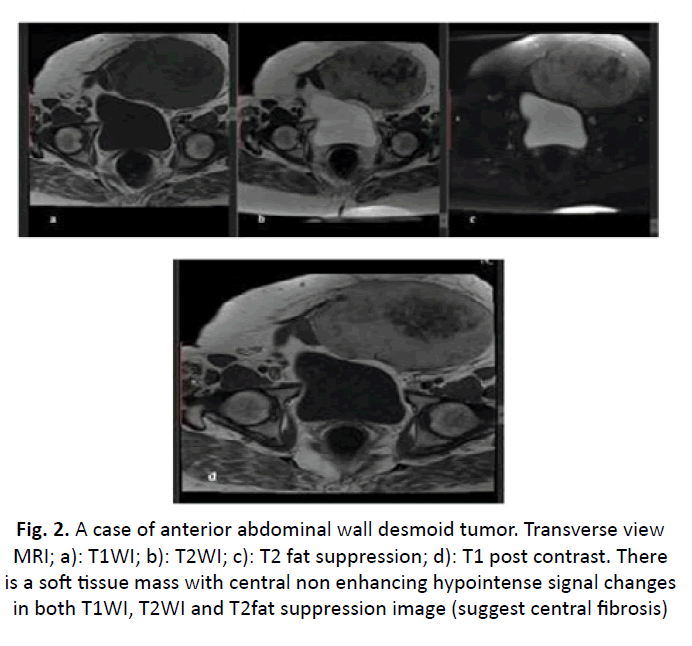
Figure 2: A case of anterior abdominal wall desmoid tumor. Transverse view MRI; a): T1WI; b): T2WI; c): T2 fat suppression; d): T1 post contrast. There is a soft tissue mass with central non enhancing hypointense signal changes in both T1WI, T2WI and T2fat suppression image (suggest central fibrosis)
Tab. 5. Correlation between the study variables and STTs (n=37)
| Variables | STTs Malignant (n=22) Benign (n=15) | p-value | ||
|---|---|---|---|---|
| Mean ± SD/No (%) | ||||
| Age | 43.3 ± 7.65 | 37.8 ± 8.1 | 0.031 | |
| M | 5 (13.5) | 7 (18.9) | ||
| Gender | F | 17 (45.9) | 8 (21.6) | 0.164 |
| Head and neck | 3 (8.1) | 5 (13.5) | ||
| Upper limbs | 0 | 3 (8.1) | ||
| Lower limbs | 13 (35.1) | 4 (10.8) | ||
| Sites | Trunk | 6 (16.2) | 3 (8.1) | 0.04 |
| <5 cm | 2 (5.4) | 6 (16.2) | ||
| Size | ≥ 5 cm | 20 (54.1) | 9 (24.3) | 0.042 |
| Isointense | 22 (59.5) | 15 | ||
| T1W1 | -40.5 | N/A | ||
| Hyperintense | 0 | 0 | ||
| Hypointense | 1 (2.7) | 0 | ||
| Hyperintense | 17 (45.9) | 8 (21.6) | ||
| T2W1 | Very hyperintense | 0.138 | ||
| 4 (10.8) | 7 (18.9) | |||
| Present | 8 (21.6) | 7 (18.9) | ||
| Non enhancing | Non-present | 14 (37.8) | 8 (21.6) | 0.734 |
| Non | Fluids | 12 (54.2) | 4 (18.2) | 0.028 |
| enhancing | Blood | 0 | 2 (9.1) | |
| Fat | 2 (9.1) | 0 | ||
| Fibrosis | 0 | 2 (9.1) | ||
| Homogeneous | 8 (21.6) | 6 (16.2) | ||
| Enhancement | Heterogeneous | 14 (37.8) | 9 (24.3) | 1 |
| Immediate | 22 (59.5) | 10 (27) | ||
| Enhancement | Gradual | 0 | 5 (13.5) | 0.007 |
| True cut | 7 (18.9) | 1 (2.7) | ||
| Methods | Excisional | 15 (40.5) | 14 | 0.075 |
| -37.8 | ||||
| Superficial | 5 (13.5) | 4 (10.8) | ||
| Locations | Deep | 17 (46) | 11 | 0.3 |
| -29.7 | ||||
Discussion
MRI is the modality of choice for evaluating soft tissue masses regarding diagnosis, characterization and planning for effective tumor management [7].
Chung et al. and his collagenous in 2012 used morphological criteria for benign lesions like smooth well-defined margins, small size and homogeneous SI, particularly on T2WI, at that time MRI was reported to be able to differentiate >90% of benign from malignant masses [8]. DWI is a functional MRI technique and can be incorporated into routine MRI protocols with little additional scanning time, resulting in a non-invasive method for the evaluation of STTs based on their histological composition [9].
In this study, we recruited 37 patients (25 females and 15 males) with STTs, with mean and median age (38.39 ± 16.59 years, and 39 years), respectively.
In our findings, patients diagnosed with malignant STTs have higher mean age than those with benign masses, and this was statistically significant difference (p=0.031). Patient’s number in this study was less than that of the study conducted by Romeih et al., who studied 50 patients (26 females and 24 males) with a single musculoskeletal soft tissue masses, ages ranged from 1.5 to 75 years with a median of 33 years [10]. Another study by Hassanien et al. in Egypt, included 45 patients (32 females and 13 males), and ages ranged from 9 to 72 years (mean age 42 ± 18.5 years) [11]. But our patients number was more than those included in the studies made by Einarsdóttir et al. [12] and that of Van Rijswijk et al. [13] which included 29 and 23 patients respectively.
The left thigh (lower limbs) represented the common site of STTs in this study, followed by anterior abdominal wall and right thigh. In Romeih et al. study reported 30 patients had lower limb soft tissue lesions, while other lesions were detected in the upper limbs and trunk [10]. In addition, STTs distributions were statistically significant differences among different sites particularly in the lower limbs sites in malignant masses (p=0.04).
In our research, the malignant masses (22/37) were higher number than benign masses (15/37). The benign STTs recorded in 40.5%, while malignant tumors were found in 59.5% of patients. That is agreement with the results of Romeih et al. [10] (benign 44% vs. malignant 56%), but disagree with Chung et al. [8], as 102 lesions were malignant and 164 were benign, and Lee et al. [14], as 66 lesions were non-malignant and 29 were malignant. This maybe because most of the benign masses are treated and excised depending on palpation, and US with some added CT scan without being sent for MRI (such as lipomas, abscesses and hematomas), which were excluded from our data, and the second reason is that our data were collected in the Oncology Teaching Hospital which is a referral hospital for malignant diseases management.
Histopathologically speaking, the excisional biopsy was the most common method used in 75.7% of cases in this study. Hemangioma was the most common benign STTs diagnosed in 16.2%, while the most common malignant STTs was myxoid liposarcoma in 10.8%.
In Romeih et al. study, they recorded rhabdomyosarcoma was the most common malignant STTs in 16%, whereas lipoma and fibromatosis were most common benign tumors in 8% of cases [10]. In present study, the number of small tumors (size<50 mm maximal diameter) was lower than those of large tumors (≥ 50 mm in maximal diameter) (8 cases vs. 29 cases). Of the 29 larger lesions, 20 (69%) were malignant, whereas only 2 of the small lesions (2/8) (25%) were malignant, with statistically significant association between larger lesion sizes and the probability of malignancy (p-value=0.042). MRI of Chung et al. study showed that 125 lesions were small and 141 were large (≥50 mm). Of the 125 small lesions, 31 (25%) were malignant and 94 (75%) were benign, and of the 141 large lesions, 71 (50%) were malignant and 70 (50%) were benign with statistically significant correlation (p<0.0001) [8], which are not consistent with our results, this could be due to the late presentation of our patients. Most of the STTs were deeply located (deep to the superficial fascia) in our study (28/37) (75.7%). Most of the malignant lesions were deeply located (18/22) (82%), only 18% of them were superficially located. This is consistent with results of Chung et al., who found that 169 lesion were deep (63%) and 97 (36%) were superficial [8]. They found that 27 of the 102 malignant lesions (27%) were superficial, and that 27 of the 97 superficial tumours (28%) were malignant, and 94 of the 164 benign lesions (56%) were classified as deep, with significant differences (p=0.0076).
Regarding MRI signal characteristics, all the masses showed hypointense or isointense signal changes in T1WI (as compared to the surrounding muscle signal) whereas in T2W1 sequences, the intermediately hyperintense signal was figured in most of the cases (67.6%) and the very hyperintense signal found in 29.7%, only one case showed hypointense signal in T2WI which was an one case of myeloid sarcoma (chloroma).
Most malignant STTs showed T2WI hyperintense signal as in (17/22) cases, while benign masses were 8 cases, with no significant difference. This could be explained by a multivariate statistical analysis of 10 imaging parameters done by De Schepper et al. individually and in combination, showed that high SI on T2WI, diameter >33 mm and heterogeneous SI on T1 weighted MR images predicted malignancy with the highest sensitivity [15].
In our study, the homogeneous enhancement was lower than the heterogeneous enhancement with the areas of nonenhancement were characterized according to the signal intensity in T1WI, T2WI and T2 fat suppression images into either fluid (hypointense signal in T1WI and hyperintense signal in T2WI with no suppression in fat suppression images), fat (hyperintense in both T1 and T2WI that suppressed in fat suppression image), blood (hyperintense in T1WI with variable signal in T2WI with no suppression in fat suppression sequence) and fibrosis (hypointense in both T1 and T2WI). Fluid non enhancing component was figured in 68.2% with the majority were malignant masses with significant association (p=0.028).
In reviewing previous studies [16-25], we found the evaluation of MR images by experienced radiologists with a centralized approach has been found to yield better diagnoses of soft-tissue tumours [20]. Most of soft tissue tumors are T1 isointense or hypointense and T2 hyperintense in signal intensity. The presence of T1 hyperintensity or T2 hypointensity in soft tissue tumors is occasionally found and helpful in differential diagnosis when present [4]. The T2- hypointense element can be a clue of some benign soft tissue tumors such as tenosynovial giant cell tumor, fibromatosis, and desmoplastic fibroblastoma [4,20]. In our cases we observed the T2 hypointense non enhancing component (regarded as fibrosis) in 2 (9.1%) and both were benign lesions (one fibromatosis and one myxoid neurofibroma). Fluid-containing lesions exhibit very high signal intensity on T2- weighted images, which allow the specific diagnosis of cystic masses, such as ganglion or bursitis [4]. As a result the contrast enhancement is helpful to differentiate these bright T2- weighted signal intensity lesions, and also showed the vascularity of soft tissue tumors [4]. As a result the contrast enhancement is helpful to differentiate these bright T2-weighted signal intensity lesions, and also showed the vascularity of soft tissue tumors [4].
The definite malignant indicators are distant metastasis and adjacent organ invasion. The likelihood of malignancy also increase with the presence of tumor necrosis, neurovascular encasement, and bone invasion [4]. The benign tumors tend to be well delineated and some malignant tumors have ill-defined margins [25]. Most soft tissue tumors have well-defined margins regardless of whether they are benign or malignant, to solved issue the administration of a contrast agent provide further information on the MR imaging characteristics of soft tissue tumors; but it does not permit the discrimination between benign and malignant lesions when evaluated qualitatively [4]. Dynamic contrast enhancement MR imaging used to differentiate malignant from benign STTs [6].
Kang et al. concluded that the combination of the three parameters arranged in order resulted in a higher diagnostic value for malignancy which were signal intensity (heterogeneity on T2-weighted images), size (≥ 50 mm), and depth (deep relative to the superficial investing fascia) [4].
Recently, Lee et al. studied 30 male and 36 female patients of non-malignant with a mean age of 45.6 years (range 12-80 years), while the malignant group comprised 19 male and 10 female patients with a mean age of 50.0 years (range 10085 years), and they detected 29 cases in the thighs, 21 in the arms, 12 in the hands, 12 in the shoulders, 8 in the feet, 7 in the trunks, and 6 in the pelvis [14].
Conclusion
Hemangioma was the commonest benign STT diagnosed, while the common malignant STT was myxoid liposarcoma. Most malignant masses showed a T2WI intermediate hyperintense signal. Gradual enhancement in dynamic contrast study gives definite diagnosis of benignity of the soft tissue masses (hemangiomas).
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors declared no potential conflicts of interest.
References
- Enzinger F, Weiss S. Soft Tissue Tumors. 2nd ed. St. Louis: Mosby. 1988;1-18.
- Grainger G, Adam A, Dixon A. Grainger and Allison’s Diagnostic Radiology: A text book of medical imaging 6th edition. Grainger and Allison's Diagnostic Radiol. 2014.
- Wu JS, Hochman MG. Soft-tissue tumors and tumor like lesions: a systematic imaging approach. Radiol. 2009;253:297-316.
- Kang HS, Hong SH, Choi JY, Yoo HJ. Oncologic Imaging: Soft Tissue Tumors. Radiol. 2017.
- De Schepper AM, De Beuckeleer L, Vandevenne J, Somville J. Magnetic resonance imaging of soft tissue tumors. Eur Radiol. 2000;10:213-223.
- Calleja M, Dimigen M, Saifuddin A. MRI of superficial soft tissue masses: analysis of features useful in distinguishing between benign and malignant lesions. Skelet Radiol. 2012;41:1517-1524.
- Ahlawat SH, Fayad LM. De novo assessment of pediatric musculoskeletal soft tissue tumors:beyond anatomic imaging. Pediatrics 2015;136:194-201.
- Chung WJ, Chung HW, Shin MJ, Lee SH, Lee MH, et al. MRI to differentiate benign from malignant soft-tissue tumours of the extremities: a simplified systematic imaging approach using depth, size and heterogeneity of signal intensity. Br J Radiol. 2012;85:e831-e836.
- Zou Y, Wang QD, Zong M, Zou YF, Shi HB. Apparent diffusion coefficient measurements with diffusion-weighted imaging for differential diagnosis of soft-tissue tumor. J Can Res Ther 2016;12:864-870.
- Romeih M, Raafat T, Khalaf M, Sallam K. The diagnostic value of diffusion-weighted magnetic resonance imaging in characterization of musculoskeletal soft tissue tumors. Egypt J Radiol Nucl Med. 2018;49:400-407.
- Hassanien OA, Younes RL, Dawoud RM. Diffusion weighted MRI of soft tissue masses: Can measurement of ADC value help in the differentiation between benign and malignant lesions?. Egyp J Radiol Nuc Med.2018; 49:681-688.
- Einarsdóttir H, Karlsson M, Wejde J, Bauer HC. Diffusion- weighted MRI of soft tissue tumors. Eur Radiol. 2004;14:959- 963.
- Benassi MS, Rimondi E, Balladelli A, Ghinelli C, Magagnoli G, et al. The role of imaging for translational research in bone tumors. Eur J Radiol. 2011;82:2115-2123.
- Lee JH, Kim HS, Yoon YC, Seo WS, Cha MJ et al. Characterization of small, deeply located soft-tissue tumors: Conventional magnetic resonance imaging features and apparent diffusion coefficient for differentiation between non- malignancy and malignancy. PLoS One. 2020;15:e0232622.
- Calleja M, Dimigen M, Saifuddin A. MRI of superficial soft tissue masses: analysis of features useful in distinguishing between benign and malignant lesions. Skelet Radiol. 2012;41:1517-1524.
- Liu L, Wu N, Ouyang H. Diagnostic value of delineating deep fascia in distinguishing between benign and malignant soft- tissue tumors in lower limbs using 3.0 T magnetic resonance imaging. J Magn Reson Imaging. 2011;33:173-179.
- Mirowitz SA, Totty WG, Lee JK. Characterization of musculoskeletal masses using dynamic GdDPTA enhanced spin-echo MRI. J Comput Assist Tomogr. 1992;16:120-125.
- Van Rijswijk CS, Kunz P, Hogendoorn PC, Taminiau AH, Doornbos J, et al. Diffusion-Weigthe MRI in the characterization of soft tissue tumors. J Magn Reson Imaging. 2002;15:302-307.
- Benassi MS, Rimondi E, Balladelli A, Ghinelli C, Magagnoli G, et al. The role of imaging for translational research in bone tumors. Eur J Radiol. 2011;82:2115-2123.
- Gielen JL, De Schepper AM, Vanhoenacker F, Parizel PM, Wang XL, et al. Accuracy of MRI in characterization of soft tissue tumors and tumor-like lesions. A prospective study in 548 patients. Eur Radiol. 2004;14:2320-2330.
- Jeon JY, Chung HW, Lee MH, Lee SH, Shin MJ. Usefulness of diffusion-weighted MR imaging for differentiating between benign and malignant superficial soft tissue tumours and tumour-like lesions. Br J Radiol. 2016; 89:20150929.
- Song Y, Yoon YC, Chong Y, Seo SW, Choi YL, et al. Diagnostic performance of conventional MRI parameters and apparent diffusion coefficient values in differentiating between benign and malignant soft-tissue tumours. Clin Radiol. 2017; 72: e691- e610.
- Subhawong TK, Jacobs MA, Fayad LM. Insights into quantitative diffusion-weighted MRI for musculoskeletal tumour imaging. AJR 2014;203:560-572.
- Pekcevik Y, Kahya MO, Kaya A. Characterization of Soft Tissue Tumors by Diffusion-Weighted Imaging. Iran J Radiol. 2015;12:e15478.
- De Schepper AM, Bloem JL. Soft tissue tumors: grading, staging, and tissue-specific diagnosis. Top Magn Reson Imaging. 2007;18:431-444.

