Research Article - Onkologia i Radioterapia ( 2022) Volume 16, Issue 1
Neoadjuvant FOLFIRINOX followed by gemcitabine based chemoradiation as a treatment paradigm for Borderline resectable pancreatic adenocarcinoma
Rasha Abd El-Ghany Khedr1*, Mohamed Ghazaly2, Amira Khedr3 and Mohamed Fathy Sheta12Department of Surgery, Faculty of Medicine, Tanta University Hospital, Egypt
3Department of Radio-Diagnosis and Medical Imaging, Faculty of Medicine, Tanta University Hospital, Tanta University, Egypt
Rasha Abd El-Ghany Khedr, Department of Clinical Oncology, Faculty of Medicine, Tanta University Hospital, Tanta University, Egypt, Email: rasha.khedr@med.tanta.edu.eg
Received: 06-Dec-2021, Manuscript No. M-49165; Accepted: 12-Jan-2022, Pre QC No. P-49165; Editor assigned: 08-Dec-2021, Pre QC No. P-49165; Reviewed: 31-Dec-2021, QC No. Q-49165; Revised: 12-Jan-2022, Manuscript No. R-49165; Published: 17-Jan-2022
Abstract
Background: The patient’s outcome for Borderline Resectable Pancreatic Adenocarcinoma (BRPC) is dismal. We aimed to evaluate FOLFIRINOX efficacy/toxicity as a neoadjuvant chemotherapy followed by gemcitabinebased chemoradiation in (BRPC).
Methods: 23 chemotherapy/radiotherapy-naïve (BRPC) patients received six months of biweekly FOLFIRINOX chemotherapy. After FOLFIRINOX (12 times), protocol-based concurrent gemcitabine/IMRT external beam radiation therapy was delivered. Gemcitabine was administered on days (1/8/22 and 29). One month later, patients without progressive disease or unacceptable toxicity continued treatment for additional 2 cycles of gemcitabine infusions. The primary endpoint was R0 resection rates. Secondary endpoints were the Overall Response Rate (ORR), progression-free survival, overall survival, and toxicity.
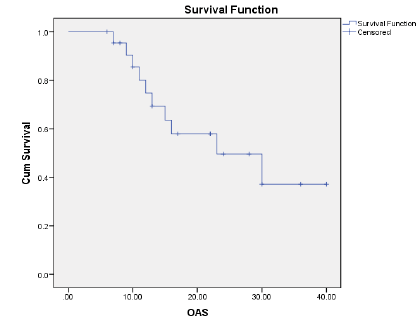
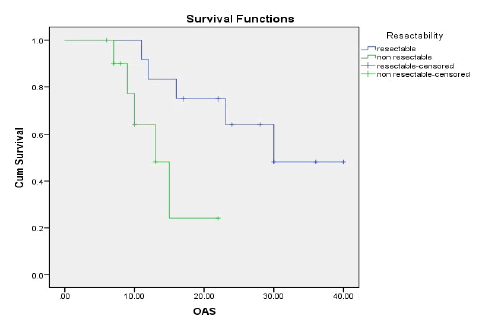
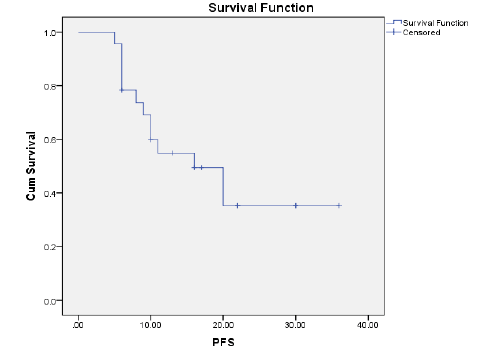
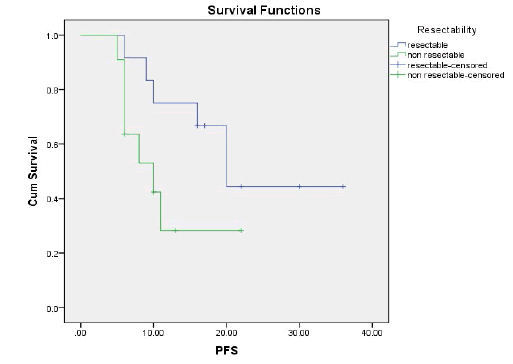
Results: The ORR was 43.5% and the disease control rate was 82.1%. Nine patients had stable disease and 4 patients had disease progression. The resection rate was 60.9%, with R0 resections at 43.5%. Median PFS and OS were 16 and 23 months, respectively.1-year and 2-year OS rates were 74.7% and 49.6% respectively. 1-year and 2-year PFS rate was 54.9% and 35.3% respectively. Neutropenia (43.5%), was and Diarrhoea (17.4%), nausea (39.1%) were the most common grade (3-4) haematological and nonhaematological toxicity, respectively.
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Conclusion: FOLFIRINOX followed by gemcitabine-based chemoradiation, was a more efficient regimen with a manageable toxicity profile in (BRPC).
Keywords
borderline resectable pancreatic cancer, FOLFIRINOX, gemcitabine based chemo radiation
Introduction
Carcinoma of the pancreas is a lethal malignancy [1-3]. Over the last few decades, it has a markedly increased incidence and ranked as the 7th leading cause of cancer-related deaths [4]. In the United States, the estimated newly reported cases and deaths from pancreatic cancer were about 55,440 and 44,330 respectively, in 2018 [5]. Cancer of the exocrine pancreas has been traditionally associated with low resectability [6-9]. Poor prognosis [2, 3,10,11], rarely curable and has a 5-year overall survival rate of 8% and a 10-year Overall Survival (OS) of 3% for all the stages [2,12]. Improvements in imaging technology, including positron emission tomographic scans, endoscopic ultrasound examination, magnetic resonance imaging scans, spiral computed tomographic scans, and laparoscopic staging can help to diagnose and identify patients with diseases that are not prone to resection [2, 7, 10, 13].
Patients with pancreatic cancer, at any stage, would be considered as appropriate candidates for the clinical trials, due to the well documented inadequate response to the conventionally used therapeutic modalities including radiation therapy, surgery, and chemotherapy [1, 3, 6, 7, 12, 14].
Patients with Borderline Resectable Pancreatic Cancer (BRPC) and locally advanced unresectable pancreatic cancers are anatomically characterized by the involvement extent of major vessels, which is likely associated with positive resection margin [15]. Several clinical trials have proposed that preoperative chemoradiotherapy for potentially resectable tumours helps to improve local recurrence and survival in resected patients [1, 7, 12, 13]. The 5-year OS was (20%) for patients undergoing resection, which may be improved up to (32%) in patients achieving complete resection and (40%) in those with nodenegative disease [12]. Thus, if the tumour is actually localized to the pancreas, the highest cure rate is recorded; however, unfortunately, only less than (20%) of patients are at this stage of the disease [14].
Several studies have recommended preoperative chemoradiotherapy for the locally advanced tumours, that were subsequently could be resected [1, 10, 12,13,16].
Previously, in a multi-institutional phase 2 study that was conducted on patients with pancreatic cancer to evaluate the neoadjuvant oxaliplatin and gemcitabine together with radiation therapy in patients with pancreatic cancer. They reported a resection rate of 63% for all treated patients, with 53% for R0 resections. Kim and his colleagues reported median survival of (18.2) months for all patients and (27.1) months, for resected patients [15].
Recently, In 2020, in the phase 2 trial on patients with (BRPC), neoadjuvant FOLFIRINOX and Intensity-Modulated Radiation Therapy (IMRT) concurrent with fixed-dose/rate gemcitabine, significantly improved the median OS for resected patients, with a median value of (37.1) months [13].
There was an amazing improvement in the magnitude of median OS, but it came with a price. Notably, there was an increased exposure and duration to local therapy intensification and systemic treatment [13]. However, the clinical evidence of significant activity in (BRPC) stimulated us to conduct this exploratory study. In this study, we investigate the efficacy of neoadjuvant FOLFIRINOX followed by gemcitabine when used concurrently with IMRT external beam radiotherapy as first-line therapy in (BRPC) patients.
Patients and Methods
Patient Eligibility Criteria
This phase II trial was carried out from January (2017) to January (2020). Twenty-three chemotherapy and radiotherapy-naïve patients with, confirmed measurable (BRPC) (BRPC stage has been defined by, the National Comprehensive Cancer Network [9], by quantification of the degree of tumour involvement with its surrounding arteries/ veins on imaging tumour involvement of or portal vein and the superior mesenteric more than (180°) without deformity, venous involvement less than (180°) with deformity, or short segment venous occlusion; celiac/superior mesenteric arteries contact of less than (180°), any common hepatic artery involvement, that is amenable to reconstruction; or direct abutment of the hepatic artery in absence of celiac axis extension) were enrolled.
Inclusion criteria
Includes Chemotherapy/radiotherapy-naïve, (18-70) years old patients; Eastern Cooperative Oncology Group (ECOG) performance status of (0-1); measurable borderline resectable disease; adequate bone marrow reserve (WBC count ≥3.5 × 109/L, ANC count ≥ 1.5 × 109/L, platelets ≥ 100 × 109/L, and haemoglobin ≥ 10 g/dL), preserved renal functions (creatinine clearance ≥ 60 mL/min) and preserved liver functions (transaminases<(2) × upper normal limit, and serum bilirubin level< (1.5) mg/dL).
Exclusion criteria
Includes symptomatic heart failure, severe arrhythmia, peripheral neuropathy, prior chemotherapy or radiotherapy, pregnant or lactating mothers, active infection, previous history of hypersensitivity reactions, any other uncontrolled medical problems or other malignancy.
Design of the study
This is a single-arm prospective (phase II) study of a single institution. Protocol approval was given by the Ethics Committee. Prior to initiation of any treatment; an informed consent was signed by all patients.
Pre-treatment evaluation
Pre/on-treatment close monitoring consisted of detailed medical history, physical examination, routine laboratory studies, pelvic and abdominal ultrasound, CT-scan of the pelvis, abdomen, and chest and (CA19.9 and CEA) measurement. Prior treatment, histologic, or cytological evidence of the pancreatic adenocarcinoma was documented in all patients.
Treatment plan and dose modification
Eligible patients received biweekly FOLFIRINOX (oxaliplatin (85) mg/m2 given as a 2-hour intravenous IV infusion, followed immediately by leucovorin 400 mg per square meter, given as a 2-hour IV infusion, with the addition, after 30 minutes, of irinotecan 180 mg per square meter, administered as a 90-minute IV infusion was followed immediately by fluorouracil 400 mg per square meter, administered by IV bolus, followed by a continuous IV infusion of 2400 mg per square meter over 46 hours, repeated every 2 weeks for 6 months of chemotherapy). Patients without Progressive Disease (PD) or unacceptable toxicity continued treatment up to 12 times over 6 months. Adequate hydration, anti-emetic therapy as well as steroids were secured for all patients. Antibiotics and growth factors, as a granulocyte-colony stimulating factor (G-CSF), were given to patients, guided by their continuous clinical evaluation.
Dose adjustment of FOLFIRINOX
Decisions were taken biweekly to modify the doses of chemotherapy, withhold treatment or progress with the schedule. Full biweekly doses of FOLFIRINOX regimen were given only if the absolute granulocyte count (AGC) was >1,000 cells/μl, platelets were > 100,000 cells/μl, and non-hematologic toxicities were ≤ grade 2. If the AGC ranged between (500-1,000) cells/ μl or the platelet count ranged between (50,000-100,000) cells/ μl, the FOLFIRINOX regimen dose was reduced by 25%. FOLFIRINOX regimen dose was reduced by 50% for grade 3 non-hematologic toxic effects. If the AGC was <500 cells/μl, the platelet count was <50,000 cells/μl and/or the non-hematologic toxic effects was grade 4, the FOLFIRINOX regimen dose was withheld, and the patient was reevaluated the following week.
Radiotherapy and gemcitabine administration
After 6 months of FOLFIRINOX, protocol-based concurrent gemcitabine IMRT external beam radiation therapy was delivered at 2.0 Gy per fraction, to a total dose of the mean planning target volume of 50.0 Gy if possible, in 25 fractions. Gemcitabine was administered on days 1, 8, 22, and 29 (1000 mg/m2 infused over 100 minutes).
Patients were treated with IMRT external beam radiation therapy. The radiotherapy field for IMRT encompassed the Gross Tumour Volume (GTV) included primary tumour and regional involved lymphatics identified on the pretreatment CT scan, including the porta hepatis, celiac axis, and superior mesenteric vessels (if involved). The Clinical Target Volume (CTV) included the GTV plus a 0.5 cm. The planning target volume (PTV) included the CTV plus 0.5 cm.
Evaluation during concurrent gemcitabine-IMRT external beam radiation therapy
During therapy, patients were assessed weekly via a directed history as well as physical examination. The occurrence and detailed nature of any adverse events, during treatment, were documented. Before each dose of gemcitabine, a full blood count was conducted. Other levels of blood chemistry were closely monitored as clinically indicated (alkaline phosphatase, bilirubin, aspartate transaminase, alanine transaminase, creatinine, blood urea nitrogen, calcium, phosphorus, glucose, albumin, total protein and electrolyte). One month after completion of protocol-based concurrent gemcitabine-IMRT external beam radiation therapy treatment monitoring consisted of a CT-scan and/or MRI of the abdomen and pelvis. Patients without PD or unacceptable toxicity continued treatment for another additional 2 cycles of gemcitabine infusions to complete neoadjuvant protocol.
Restaging
After treatment completion, all patients were re-evaluated by CTscan and/or MRI of the abdomen and pelvis to radiographically document tumour response. Based on the assessment of CT images taken at the time of restaging, surgery was considered in patients whose disorder was assumed to be technically resectable after therapy completion.
Patient Assessment
Assessment of clinical benefit, and follow-up
Tumour response was assessed based on the Response Evaluation Criteria in Solid Tumors [17], with the overall response rate, including partial/complete response, while, the disease control rate, including partial response, complete response, and stable disease. Patients were evaluated, after treatment completion, by physical examination, abdominopelvic CT, and chest radiography, every 3-4 months. Biopsy from new recurrent disease sites was rarely carried out and was reported at the time of initial occurrence.
Assessment of toxicity
During therapy, all patients were carefully examined bimonthly via a directed history as well as physical examination. The occurrence/nature of any adverse events was reported. The toxicity grading was based upon standard terminology standards for adverse events (NCI-CTC, version 3.0) [18].
Primary and Secondary Endpoints
The primary endpoint of this study was R0 resection rates, (as defined by the absence of both microscopic/gross involvements of tumour resection margins) in (BRPC) population. Secondary endpoints were the overall response rate, OS, progression-free survival and toxicity. Disease progression was assessed from the first chemotherapy dose, and it was defined as increase in the size of a previously present disorder as documented by serial axial CT, the appearance of new local/distant metastatic disorder.
Statistical analysis
Twenty-three patients were enrolled in the current study between January 2017 and January 2020. The date of this analysis was June 2021.
OS rates were calculated, by the Kaplan-Meier method [19], from the start of biweekly FOLFIRINOX to the time of the last follow-up visit or death, using SPSS (Statistical package, version 21.0). Progression-free survival was the time elapsed from the initiation date of biweekly FOLFIRINOX to the date of the first evidence of disorder progression or death in the absence of disease progression. OS and progression-free survival were compared by the Kaplan-Meier method [19] with statistical significance evaluated by the log-rank test. Mean and Standard Deviation (SD) were calculated from quantitative data. All P values were two-tailed; a value of ≤ 0.05 was considered significant.
Results
Patient characteristics
Patient characteristics were listed in Table 1. The median age was 53 years (range, 36-68). Fourteen patients (60.8%) had performance status 1. Twelve patients (52.2%) had involved body and tail pancreatic sites. Three (13.1%) patients had a biliary stent. The median level of CA19.9 was 600 U/ml. The median maximum cross-sectional Tumour Area (TA) was 8.7 cm2.
| Patient Characteristics | No. | % |
|---|---|---|
| Sex | ||
| Male | 15 | 65.2 |
| Female | 8 | 34.8 |
| Age, years | ||
| Median | 53 | |
| Range | 36-68 | |
| ECOG performance status | ||
| 0 | 9 | 39.1 |
| 1 | 14 | 60.9 |
| Tumor location | ||
| Head | 9 | 39.1 |
| Body & tail | 12 | 52.2 |
| Overlapped lesion | 2 | 8.7 |
| Biliary stent | ||
| Yes | 3 | 13.1 |
| No | 20 | 86.9 |
| Presenting symptoms | ||
| Jaundice | 16 | 70 |
| Fatigue | 11 | 48 |
| Abdominal pain | 17 | 74 |
| Change in bowel pattern | 10 | 43.5 |
| Back pain | 9 | 39 |
| Anorexia | 7 | 30 |
| Largest axial area on CT | ||
| Median | 8.7 cm2 | |
| Range | 2.5 – 37 cm2 | |
| Level of CA19.9, U/ml | ||
| Median | 600 | |
| Range | 0 – 101 0,66 | |
ECOG:- Eastern Cooperative Oncology Group |
||
CA19.9:- Carbohydrate antigen |
||
Tab.1. Baseline patient and tumour characteristics of the 23 patients with borderline resectable pancreatic cancer
Treatment administration
A total of 114 FOLFIRINOX chemotherapy cycles were administered. Patients were treated with a median number of 4 cycles of FOLFIRINOX (range 3-6 cycles), with dose modifications (according to criteria of dose adjustment mentioned previously in patients and methods) in 48.2% (55/114) of all FOLFIRINOX cycles.
Four patients (17.4%) had >3 dose delay of gemcitabine during concurrent gemcitabine-IMRT external beam radiation therapy. Two (9%) patients had radiotherapy interruptions due to toxicity. Fifteen patients (65.2%) complete 2 cycles of gemcitabine post radiation therapy.
Patients’ response to this regimen
The overall response rate (CR+PR) was 43.5% (10/23) of all patients and the disease control rate (CR+PR+SD) was 82.1% (19 patients). Nine patients (39.1%) had stable disease and 4 patients (17.4%) had disease progression (Table 2).
| Evaluable patients | N=23 | |
|---|---|---|