Case Report - Onkologia i Radioterapia ( 2023) Volume 17, Issue 7
Optic neuropathy in a patient treated for glioblastoma:Side effect of bevacizumab or radiotherapy?
Imane Stitou1*, Mohammed Ismaili1, Fatima Oussi2, Khaoula Alaoui Ismaili1, Lamiae Amaadour1, Karima Oualla1, Zineb Benbrahim1, Samia Arifi1 and Nawfel Mellas12Radiology department, University Hospital Centre Hassan II, Fes, Morocco
Imane Stitou, Oncology department, University Hospital Centre Hassan II, Fes, Morocco, Tel: +964 790 299 0346, Email: imanest86@gmail.com
Received: 26-Apr-2023, Manuscript No. OAR-23-97094; Accepted: 01-Jun-2023, Pre QC No. OAR-23-97094 (PQ); Editor assigned: 29-Apr-2023, Pre QC No. OAR-23-97094 (PQ); Reviewed: 15-May-2023, QC No. OAR-23-97094 (Q); Revised: 19-May-2023, Manuscript No. OAR-23-97094 (R); Published: 06-Jun-2023
Abstract
Glioblastoma is the most common primary brain tumour in adults. It’s often rapid evolution requires a multidisciplinary management. Surgery, radiotherapy and Temozolomide are the first-line treatments. At the time of disease recurrence, few treatment options are available. Bevacizumab has been considered the breakthrough treatment for this disease by leading to improved progression-free survival.
Optic neuropathy is an uncommon but well-documented complication of radiation therapy for brain tumours. Contrary to bevacizumab, its relationship with the occurrence of this side effect remains controversial at this stage and requires further studies.
We report the case of a patient followed for recurrent glioblastoma who developed optic neuropathy after 18 months of treatment with bevacizumab.
Keywords
Optic neuropathy , bevacizumab, glioblastoma, radiotherapy
Introduction
Optic neuropathy is a rare complication of glioblastoma treatment. The link between optic neuropathy and radiotherapy is well established but few data address the relationship between Bevacizumab and optic neuropathy. Its diagnosis is made by exclusion and mainly by clinical analysis of the case. No treatment seems promising at this time.
Randomized studies should be conducted to better define the relationship between efficacy and safety of be vacizumab us e after radiotherapy for glioblastoma and thus be able to propose effective treatments [1-3].
Case Report
Mr B a 38-years-old patient, with no pathological antecedents, followed in the medical oncology department since 2020 for recurrence temporal glioblastoma.
Bevacizumab was initiated at the time of tumour progression, which was 6 months after the completion of radiotherapy with concomitant temozolamide. Mr B. received 60 Gy delivered in 30 fractions.
After 18 months of Bevacizumab (10 mg/kg every 2 weeks), Mr B. developed acute bilateral blindness. Pseudo progression was ruled out with magnetic resonance. The ophthalmic examination revealed that visual acuity was 0/10 with bilateral mydriasis and photo motor reflex abolished bilaterally. The Fundus examination was normal. MRI did not show any signal abnormalities in the peripheral optic tracts related to an inflammatory or infectious or neoplastic origin and no infiltration by the already known process and the CSF results excluded the diagnosis of carcinomatous meningitis or autoimmune demyelination (Figures 1 and 2). Therefore, the toxic origin was retained considering the context and the normality of the explorations. The patient was hospitalized and put on a corticosteroid infusion at a dose of 1 g/3 h for 5 days without any improvement of his symptoms.
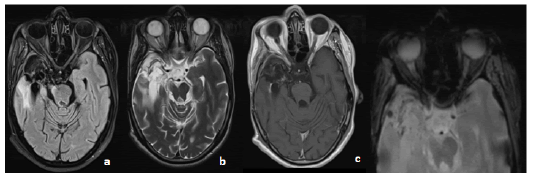
Figure 1: MRI in axial slices in T2 Flair a. T2 b. T1 injected c. Gradient echo sequences showing a normal aspect of both nerves and the optic chiasm
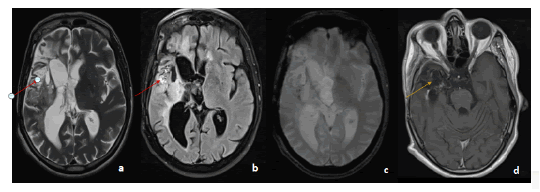
Figure 2:MRI in axial slices in T2 a. T2 Flair b. gradient echo c. T1 injected sequences: showing the presence of an intra-axial tumoral process in T2 and Flair hyper signal (red arrow), containing hemorrhagic stigmata, enhanced after injection of PDCI. Right temporal porencephalic cavity associated with retractile ventricular dilatation (yellow arrow)
Discussion
Glioblastoma is the most aggressive malignant brain tumour in adults and is almost always fatal. The prognosis for these patients stays poor despite optimal treatment. At the time of relapse, few treatment options are available.
Since 2007, antiangiogenic therapy using Bevacizumab alone or in combination with Irinotecan has emerged as a promising development in the treatment of recurrent GBM. Indeed, in this phase II trial, BV alone or in combination with Irinotecan improved the progression-free survival and objective response rate with good tolerance profile [4].
Bevacizumab is a humanized monoclonal antibody that inhibits VEGF that is a regulator of angiogenesis implicated in pathologic angiogenesis associated with tumour growth. It is highly expressed in glioblastoma, and overexpression correlates with high-grade malignancy and poor prognosis [5].
The most commonly described side effects of Bevacizumab are arterial and venous thrombosis, Hemorrhage, high blood pressure, proteinuria, and gastrointestinal perforation. Vision loss has not been previously confirmed [4]. In a retrospective study published in 2009, 6 patients treated for glioblastoma developed optic neuropathy after treatment with Bevacizumab. The authors of this article concluded that Bevacizumab was involved in the occurrence of this complication given the normality of the complementary examinations and the delay between radiotherapy and the latter. This was explained by the possibility of the occurrence of probable arterial thrombosis or VEGF upregulation and neovascularization after radiotherapy with delayed ischemia after Bevacizumab [6].
The clinical presentation of our patient, as well as the results of the MRI (which was normal), were similar to those of the abovementioned patients, supporting the hypothesis of the neurotoxicity of bevacizumab after irradiation of a gliobalstoma.
However, the relationship between radiotherapy and optic neuropathy is well established. It can occur if cranial nerves are included in the radiation treatment field. In a study published in 2009 by Demizu and al, 11% of patients followed for tumors of the ENT sphere and the base of the skull presented an optic neuropathy after irradiation with a very variable delay of onset (between 3 and 18 months) [7]. In another series reported by Stafford et al, cases of radiation-induced optic neuropathy were also described in 2% of cases in a series of 215 patients [8]. According to Seregard et al, the onset of radiation-induced optic neuropathy was noted from cumulative doses of 50 Gy. This risk increases with increasing fractional doses [9]. Other risk factors may be involved in the occurrence of radiation-induced neuropathy, including compression of the otic-nerve by the tumour, or concomitant administration of certain chemo toxic agents with radiotherapy [10-11].
None of the treatments studied have been shown to be effective in the treatment of optic neuropathy. The uses of corticoids, anticoagulants or even hyperbaric oxygen therapy have been tested on small samples and have not shown any improvement in vision.
Conclusion
The involvement of Bevacizumab remains unknown. Although the etiology and mechanism remain unclear, the association between this rare event and bevacizumab is questionable. Studies should be carried out in this sense to affirm or confirm the involvement of the latter in the appearance of optic neuropathy after treatment for glioblastoma.
Ethics
Written informed consent was obtained from the patient for publication of this case report.
Conflict Of Interest Statement
There are no conflicts of interest to declare by any of the authors by this case report.
References
- Stupp R, Mason WP. Radiotherapy plus concomitant and adjuvant temozolomide for patients with newly diagnosed glioblastoma. N Engl J Med; 352:987-996.
- Fu DB, Alexandru D, Curticiu DM, Fu Y, Bota DA. Two patients with brain tumors who received bevacizumab and radiotherapy: optic neuropathy and quality-of-life issues. J Adv Pract Oncol. 2013; 4:252.
- Nagane M, Nishikawa R, Narita Y, Kobayashi H, Takano S, et al. Phase II study of single-agent bevacizumab in Japanese patients with recurrent malignant glioma. Jpn J Clin Oncol. 2012; 42:887-895.
- Vredenburgh JJ, Desjardins A, Herndon JE, Dowell JM, Reardon DA, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer res. 2007; 13:1253-1259.
- Godard S, Getz G, Delorenzi M, Farmer P, Kobayashi H, et al. Classification of human astrocytic gliomas on the basis of gene expression: a correlated group of genes with angiogenic activity emerges as a strong predictor of subtypes. Cancer Res. 2003; 63:6613-6625.
- Sherman JH, Aregawi DG, Lai A, Fathallah-Shaykh HM, Bierman PJ, et al. Optic neuropathy in patients with glioblastoma receiving bevacizumab. Neurology. 2009; 73:1924-1926.
- Demizu Y, Murakami M, Miyawaki D, Niwa Y, Akagi T, et al. Analysis of vision loss caused by radiation-induced optic neuropathy after particle therapy for head-and-neck and skull-base tumors adjacent to optic nerves. Int J Radiat Oncol * Biol* Phys. 2009;75:1487-1492.
- Scott L Stafford , Bruce E Pollock , Jacqueline A Leavitt , Robert L Foote, Paul D Brown, et al. A study on the radiation tolerance of the optic nerves and chiasm after stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2003; 55:1177-1181.
- Seregard S, Pelayes DE, Singh AD. Radiation therapy: posterior segment complications. Ophthalmic Radiat Ther. 2013;52:114-123.
- Arrieta O, Michel Ortega RM, Villanueva-Rodríguez G, Serna-Thomé MG, Flores-Estrada D, et al. Association of nutritional status and serum albumin levels with development of toxicity in patients with advanced non-small cell lung cancer treated with paclitaxel-cisplatin chemotherapy: a prospective study. BMC canc. 2010; 10:1-7.
- Sanderson PA, Kuwabara T, Cogan DG. Optic neuropathy presumably caused by vincristine therapy. Am J Ophthalmol. 1976; 81:146-150.