Research Article - Onkologia i Radioterapia ( 2023) Volume 17, Issue 7
Percutaneous trans-hepatic local sclerotherapy of symptomatic giant liver hemangioma
Rafid Abdulrazzaq Abdulazeez1*, Rana T. Mehsen2 and Zaid Hadi Kadhim32Departments of Pathological Processes & Therapeutics, American University School of Medicine, Aruba
3Fellowship in interventional radiology. Consultant diagnostic and interventional radiologist, Aruba
Rafid Abdulrazzaq Abdulazeez, Department of Premedical Sciences, and Nutritional, Biochemical & Molecular Sciences, American University School of Medicine, Aruba, Email: rafid76@yahoo.com
Received: 16-Feb-2023, Manuscript No. OAR-22-89329; Accepted: 11-Jul-2023, Pre QC No. OAR-22-89329 (PQ); Editor assigned: 28-Mar-2023, Pre QC No. OAR-22-89329 (PQ); Reviewed: 12-Apr-2023, QC No. OAR-22-89329 (Q); Revised: 10-Jul-2023, Manuscript No. OAR-22-89329 (R); Published: 17-Jul-2023
Abstract
Background: The hepatic haemangioma is a common benign tumour of the liver that is diagnosed incidentally by radiology. Different treatment options are available in treatment of hepatic haemangioma with different advantages and disadvantages. Aim of study: To evaluate the role of percutaneous sclerotherapy with bleomycin in the treatment of giant hepatic haemangioma.
Patients and methods: A clinical prospective follow up study carried out in the Ghazi Al-Hariry Teaching Hospital/ Medical City Complex in Baghdad City-Iraq through the period of six months 1st of December, 2021 to 31st of May, 2022 on convenient sample of fifteen patients with symptomatic liver haemangioma. The diagnosis of symptomatic liver haemangioma was done by Radiologist by computerized tomography scan. The procedure was performed injecting 60 IU bleomycin under ultrasound provision.
Results: There was a highly significant decline in Craniocaudally diameter of haemangioma 6 months after injection (p<0.001). A highly significant decline was observed in transverse diameter of haemangioma 6 months after injection (p<0.001). There was a highly significant decline in anteroposterior diameter of haemangioma 6 months after injection (p<0.001). Consequently, the volume and size of haemangioma were significantly declined 6 months after injection (p<0.001). Conclusions: The percutaneous sclerotherapy with bleomycin is new method in the treatment of giant hepatic haemangioma.
Keywords
hepatic haemangioma, percutaneous sclerotherapy, bleomycin
Introduction
The majority of benign liver tumours (73%) are cavernous haemangiomas, which may occur as often as 7.3% in autopsy series [1]. Although hepatic haemangiomas can be detected using a variety of imaging techniques such as ultrasound, Magnetic Resonance Imaging (MRI), and Computerised Tomography (CT), IV contrast-enhanced abdominal CT remains the gold standard for diagnosis. Hepatic haemangiomas are more common in middle-aged women and can grow larger during pregnancy [1,2]. Hepatic haemangiomas are often discovered incidentally during routine imaging and are typically tiny (less than 1 cm), stable, and asymptomatic. Giant haemangiomas are lesions that are 4 cm to 5 cm or larger; despite their significant intra-abdominal development, they often have no symptoms [1-3]. Patients often report right upper quadrant stomach pain, discomfort, and fullness as a result of the Glisson's capsule being stretched and inflamed. Abdominal squeezing is observed in tumours larger than 10 cm [4]. Other symptoms including nausea, early satiety, and postprandial bloating may also be caused by the placement of the liver mass, which may push against and compress nearby tissues. The following symptoms are less typical ones: fever, jaundice, dyspnea, high-output cardiac failure, and hemomobilia [5,6]. The incidence of the potentially fatal Kasabach-Merrit syndrome, which includes thrombocytopenia, disseminated intravascular coagulation, and systemic bleeding, has been observed to range from 0.3% of all HH to 26% in tumours larger than 15 cm [4,7]. Although the danger of bleeding from spontaneous or traumatic rupture is very minimal (0.47%), peripherally situated and exophytic large lesions may do so. 8. Prophylactic treatment through surgical resection or other methods has historically been the norm of care to prevent potentially serious complications, such as rupture/bleeding, thrombosis, or disseminated intravascular coagulation/consumptive thrombocytopenia. Management of giant hemangiomas is controversial (Kasabach-Merritt Syndrome) [2]. However, surgical removal of an asymptomatic lesion should not be done only for the purpose of preventing rupture [3]. In contrast, therapy should be provided to patients who have symptomatic lesions that are harming their quality of life, and the literature outlines a number of strategies that may be used in a secure and efficient manner. In comparison to postoperative recovery, blood loss, complications, hospital stays, and reduced hospital expenditures, percutaneous sclerotherapy is the preferable approach for treating large hepatic haemangiomas compared to surgical excision. The percutaneous sclerotherapy group's decrease in the maximal cross-sectional area of hepatic haemangioma is good [8-13]. The purpose of the research is to assess the effectiveness of bleomycin-infused percutaneous sclerotherapy in the management of massive hepatic haemangiomas.
Material & Methods
Current study was a clinical prospective follow up study for fifteen patients with symptomatic liver haemangioma referred to the Ghazi Al-Hariry Teaching Hospital/ Medical City Complex in Baghdad city -Iraq through the period of six months 1st of December, 2021 to 31st of May,
Inclusion criteria
1. Adult patients (age ≥ 18 years).
2. Giant haemangioma larger than 5 cm diameter.
3. Symptomatic (pain and heaviness at liver site) as evaluated by Gastroenterologist.
4. Abnormal investigation (low platelet, anemia, abnormal liver function tests).
5. Typical radiological feature of hemangioma.
Exclusion criteria
1. Pregnancy.
2. Small haemangioma
3. Asymptomatic.
4. Atypical radiological feature.
5. Elevated alpha fetoprotein.
6. Contraindication to sclerotherapy.
The data was collected directly from patients and filled in a prepared questionnaire. The following information was checked in every patient:
1. Demographic characteristics of patients with symptomatic liver hemangioma: Age and gender.
2. CT scan 3 dimensional measures of hemangioma before treatment:
3. CT scan hemangioma enhancement pattern before & after treatment
Radiologist used a dynamic CT scan to determine the hepatic hemangioma's symptoms. The Ghazi AL-Hariry Teaching Hospital or a private laboratory conducted all of the studies. Siemens 64 slice Somatom AS was the CT scanner that was utilised. Dynamic CT scan with 3-phase contrast is required for hepatic haemangioma diagnosis and follow-up criteria (arterial at 40 seconds, venous phase at 70 seconds and delayed phase at 3 minutes-5 minutes). Outpatient care was used for the surgery. Under ultrasound guidance, patients were given a single dose of a corticosteroid (8 mg ampoule of dexamethasone intravenously) and a prophylactic antibiotic (1 g of ceftriaxone intravenous) to reduce the risk of adverse responses to Bleomycin and infection, respectively. When feasible, the Haemangioma was reached via the normal liver parenchyma using a 20-gauge spinal needle and ultrasound guidance. Local anaesthesia was induced with 5 cc of 2% lidocaine. In order to determine whether the lesion communicated with the venous, portal, or biliary systems, 5 cc-10 cc of the contrast agent (Omnipaque 350; GE Healthcare, Cork, Ireland) was then slowly injected into the centre of the lesion under fluoroscopy. If any such communications were observed, the needle was then moved. Next, 10 cc of distilled water was used to dilute 60 IU of bleomycin. Then, over the course of 20 seconds to 30 seconds, the prepared Bleomycin combination was gently administered intralesionally under fluoroscopy. Patients were observed for 2 hours after the treatment to look for any early problems, including intraperitoneal haemorrhage and allergic responses. The patient was released on oral antibiotics if vital signs were stable, there was no acute discomfort, and an ultrasound for intra-abdominal free fluid or hematoma came back negative (cefixime 400 mg). Liver function tests, including AST, ALT, ALP, and bilirubin, were assessed in all patients a day after the surgery. Patients were questioned six months later to see whether they had any linked stomach symptoms to Bleomycin, as well as any respiratory problems. To assess possible treatment-induced hepatic damage, liver function tests and lung injury assessments were conducted once again
Results
In this research, 15 patients with symptomatic hepatic haemangiomas were included. Their ages ranged from 23-52 years, with a mean of 41.57 years. Of them, 6.7% were under 30 years old, 26.6% were between 30 and 39 years old, 60.3% were between 40 and 49 years old, and 6.7% were 50 years and beyond. Female patients with symptomatic hepatic haemangiomas outnumbered male patients by a ratio of 14:1 more often. (Table 1)
Tab. 1. Demographic characteristics of patients with symptomatic liver hemangioma
| Variable | Number | Percentage (%) | |
|---|---|---|---|
| Age mean ± SD (41.5 ± 7 years) | <30 years | 1 | 6.7 |
| 30 years -39 years | 4 | 26.6 | |
| 40 years-49 years | 9 | 60 | |
| ≥50 years | 1 | 6.7 | |
| Total | 15 | 100 | |
| Gender | Male | 1 | 6.7 |
| Female | 14 | 93.3 | |
| Total | 15 | 100 | |
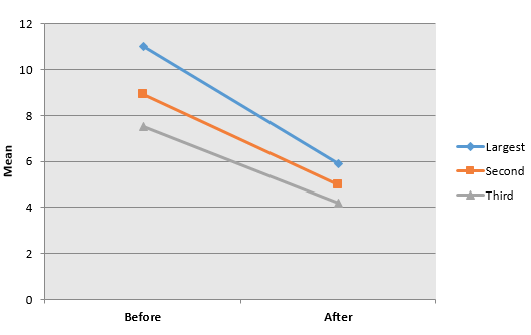
Before treatment, the CT scan measures of haemangioma revealed that first (Craniocaudal) diameter mean was (11 cm, minimum 9.2 cm and maximum 13.4 cm), second (transverse) diameter mean was (8.9 cm, minimum 6.5 cm and maximum 12.3 cm), third (anterioposterior) diameter mean was (7.5 cm, minimum 6 cm and maximum 12 cm), while mean volume of haemangioma was (779.3 cm, minimum 448.5 cm and maximum 1977.8 cm) and mean size of haemangioma was (790.9 cm, minimum 448.5 cm and maximum 1977.8 cm) (Table 2). After treatment with percutaneous local injection of 60 IU of bleomycin, the CT scan measures of haemangioma revealed that first ( Craniocaudal) diameter mean was (5.9 cm, minimum 3.5 cm and maximum 7.6 cm), second (transverse) diameter mean was (5 cm, minimum 2.4 cm and maximum 8 cm), third (anterioposterior) diameter mean was (4.2 cm, minimum 62.2 cm and maximum 6.5 cm), while mean volume of hemangioma was (148.4 cm, minimum 18.5 cm and maximum 384.8 cm) and mean size of haemangioma was (176.7 cm3 , minimum 18.5 cm3 and maximum 384.8 cm3 )(Table 2).
Tab. 2. CT scan measures of haemangioma post injection.
| Variable | Before | After | Mean difference | Reduction (%) | P- value |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | ||||
| 1st diameter (cm) | 11 ± 1.5 | 5.9 ± 1.4 | 5.07 | 47.7 | <0.001*S |
| 2nd diameter (cm) | 8.9 ± 1.7 | 5 ± 1.7 | 3.8 | 43.2 | <0.001*S |
| 3rd diameter (cm) | 7.5 ± 1.5 | 4.2 ± 1.2 | 3.3 | 44 | <0.001*S |
| Volume (cm3) | 779.3 ± 246.6 | 148.4 ± 100.9 | 630.9 | 81 | <0.001*S |
| Size (cm3) | 790.9 ± 508.3 | 176.9 ± 126.8 | 623 | 78.7 | 0.001*S |
*Paired t-test, S=Significant.
As shown in table 1 and figures 1; there was a highly significant decline in largest (Craniocaudal) diameter of haemangioma 6 months after injection (p<0.001) with reduction of 47.7% in original diameter. A highly significant decline was observed in second (transverse) diameter of haemangioma 6 months after injection (p<0.001) with reduction of 43.2% in original diameter. There was a highly significant decline in third (anterioposterior) diameter of haemangioma 6 months after injection (p<0.001) with reduction of 44% in original diameter. Consequently, the volume of haemangioma was significantly declined 6 months after injection with percutaneous local injection of 60 IU of bleomycin as compared to haemangioma volume before injection (p<0.001) with reduction of 81% in original volume. The size of haemangioma was significantly declined 6 months after injection with percutaneous local injection of 60 IU of bleomycin as compared to haemangioma size before injection (p=0.001) with reduction of 78.7% in original size.
Figure 1: CT scan diameters of hemangioma before and 6 months after percutaneous local injection of 60 IU of bleomycin.
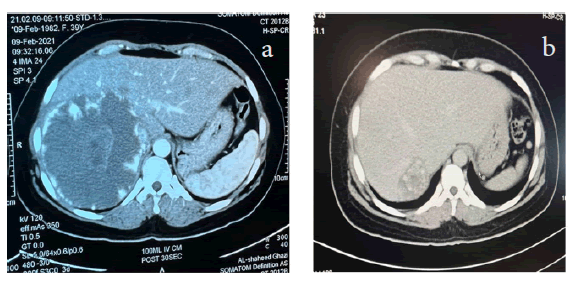
The CT scan after treatment for patients with symptomatic liver haemangioma showed commonly heterogeneous enhancement with central sparing (40%) and peripheral interrupted nodular (33.3%), and less commonly; central sparing enhancement (20%) and heterogeneous enhancement (6.7%). (Table 2, 3 and Figure 2)
Tab. 3. CT scan enhancement characteristics of patients with symptomatic liver haemangioma after treatment.
| Variable | Number | Percentage (%) |
|---|---|---|
| Peripheral interrupted nodular | 5 | 33.3 |
| Heterogenous enhancement with central sparing | 6 | 40 |
| Central sparing enhancement | 3 | 20 |
| Heterogenous enhancement | 1 | 6.7 |
| Total | 15 | 100 |
Figure 2: Dynamic CT scan a. before treatment showing giant haemangioma in right lobe with nodular peripheral enhancement. b. After treatment with percutaneous local injection of 60 IU bleomycin
Discussion
The hepatic haemangioma is found in 3%-20% of general population. Giant haemangioma is symptomatic and needs surgical intervention. The minimally invasive techniques are of great benefits as alternative to surgery 13. The current study showed that mean age of patients with symptomatic liver haemangioma was (41.5 years). This mean age is close to mean age of (45 years) reported by Mogahed et al. study in Egypt on 186 patients’ symptomatic haemangioma [14]. Liu et al. stated that age is common independent risk factor affecting growth and prevalence of liver haemangioma [15]. In current study, Female patients with symptomatic liver haemangioma were more than males with female to male ratio as 14:1. This finding is close to results of Kamyab et al. retrospective study in Iran which reported that females are highly predominant with symptomatic giant haemangioma especially at fourth decade of life [16]. Many authors revealed that liver haemangioma tumour is found commonly among women with female to male ratio reaching to 5:1 [14-18]. In current study, the CT scan findings of patients with symptomatic liver haemangioma before bleomycin treatment showed nodular peripheral enhancement in arterial phase, progressive centripetal filling in venous phase and complete filing in delayed phase. These findings are close to reports of Klotz et al. review study in France which documented that common typical CT scan features of hepatic haemangioma included cavernous haemangioma, capillary haemangioma, sclerosed haemangioma and CT scan features of giant haemangioma (thrombosis, liquefaction and fibrosis) [19]. Our study showed that all patients with symptomatic liver haemangioma received percutaneous local injection of 60 IU of bleomycin. A study conducted in Pakistan by Memon et al. on 30 patients with hepatic haemangioma reported that ultrasound-guided intralesional injection of bleomycin is effective in management of haemangioma and vascular malformation [20]. In present study, there was a highly significant decline in largest, second and third diameters of haemangioma after treatment (p<0.001) with reduction of 47.7%, 43.2% and 44% in original diameter, respectively. These findings are similar to results of Gupta et al. study in India which stated that intralesional bleomycin injection is also useful in management of ocular and periocular haemangioma [21]. Our study revealed that volume of haemangioma was significantly declined after treatment with percutaneous local injection of 60 IU of bleomycin as compared to haemangioma volume before treatment (p<0.001) with reduction of 81% in original volume. This finding is consistent with results of 22. McGahan JP et al. single-institute prospective study in Iran on twenty-eight patients with giant symptomatic hepatic haemangioma which revealed a significant decline in haemangioma CT scan volume (65.7% reduction) after ultrasound-guided bleomycin treatment [22]. In same way, the size of haemangioma was significantly declined after treatment with percutaneous local injection of 60 IU of bleomycin as compared to haemangioma volume before treatment (p=0.001) with reduction of 78.7% in original size. This finding is similar to results of Hashimoto et al study in Japan which reported that size of hepatic haemangioma was decreased (68% reduction) after implementing ultrasound guided 60 IU of percutaneous bleomycin injection [23]. Generally, our study findings regarding response of symptomatic hepatic haemangioma are similar to results of Nevesny et al. study in France which found that bleomycin is clinically and radiologically effective in management of venous and lymphatic malformations [24]. In current study, the CT scan after treatment for patients with symptomatic liver haemangioma showed commonly heterogeneous enhancement with central sparing (40%) and peripheral interrupted nodular (33.3%), and less commonly; central sparing enhancement (20%) and heterogeneous enhancement (6.7%). These findings are close to results of Ketchum et al. study in USA and Firouznia et al. study in Iran which all reported that after management of giant hepatic haemangioma with percutaneous bleomycin, the CT scan showed mainly heterogeneous enhancement with central sparing and peripheral interrupted nodular lesions indicating reducing effect of hepatic haemangioma [25, 26].
Conclusion
One novel approach to treating big hepatic hemangiomas is bleomycin sclerotherapy, which is administered through a catheter. CT scans are crucial for assessing the progression of a big hepatic hemangioma both before and after therapy. The size, volume, and diameter of a big hepatic hemangioma may be successfully reduced with percutaneous sclerotherapy with bleomycin. Women in their forties are more likely to develop a massive hepatic hemangioma than males.
References
- Bailey J, Di Carlo S, Blackwell J, Gomez D. Same day arterial embolisation followed by hepatic resection for treatment of giant haemangioma. Case Rep. 2016;1-3.
- Lisette TH, Matthanja B, Deha E, Joris JT, Ulrich HW, et al. Management of giant liver hemangiomas: an update. Expert Rev Gastroenterol. Hepatol. 2013; 7:263-268.
- Plackett TP, Lin-Hurtubise KM. Hepatic hemangiomas and parachuting. Aviat space Environ Med. 2008;79:986-988.
- Sakamoto Y, Kokudo N, Watadani T, Shibahara J, Yamamoto M, et al. Proposal of sizeâ?based surgical indication criteria for liver hemangioma based on a nationwide survey in Japan. J Hepatoâ?Biliaryâ?Pancreatic Sci. 2017; 24:417-425.
- Liu X, Yang Z, Tan H, Zhou W, Su Y. Fever of unknown origin caused by giant hepatic hemangioma. J Gastrointest Surg. 2018; 22:366-367.
- Smith AA, Nelson M. High-output heart failure from a hepatic hemangioma with exertion-induced hypoxia. J Gastrointest Surg. 2016;117:157-158.
- Miura JT, Amini A, Schmocker R, Nichols S, Sukato D, et al. Surgical management of hepatic hemangiomas: a multiâ?institutional experience. HPB. 2014; 16:924-928.
[Google Scholar] [CrossRef]
- Mocchegiani F, Vincenzi P, Coletta M, Agostini A, Marzioni M, et al. Prevalence and clinical outcome of hepatic haemangioma with specific reference to the risk of rupture: a large retrospective cross-sectional study. Dig Liver Dis. 2016; 48:309-314.
- Bajenaru N, Balaban V, SÄ?vulescu F, Campeanu I, Patrascu T. Hepatic hemangioma-review. J Med Life. 2015;8:4.
- Sharma A, Kaspar M, Siddiqui M, Kim J. Enucleation after embolization of liver failure-causing giant liver hemangioma. Am J Case Rep. 2015; 16:563.
- Zhang W, Huang ZY, Ke CS, Wu C, Zhang ZW, et al. Surgical treatment of giant liver hemangioma larger than 10 cm: a single center's experience with 86 patients. Medicine. 2015;94.
- Lin Z, Zhu X, Zhou J. Ultrasound-guided percutaneous sclerotherapy versus surgical resection in the treatment of large hepatic hemangiomas: a retrospective study. BMC surgery. 2022; 22:1-9.
- Kirnap M, Boyvat F, Boyacioglu S, Hilmioglu F, Moray G, et al. The effect of bleomycin embolization on symptomatic improvement and hemangioma size among patients with giant liver hemangiomas. Open J Gastroenterol. 2018; 8.
- Mogahed MM, Zytoon AA, Essa B, Abdellatif W, Ghanem N, et al. Natural history of hepatic hemangiomas as a guide for surgical indication. Egypt Liver J. 2020 Dec; 10:1-7.
- Liu X, Yang Z, Tan H, Xu L, Liu L, et al. Patient age affects the growth of liver haemangioma. HPB. 2018; 20:64-68.
[Google Scholar] [CrossRef]
- Kamyab AA, Rezaei-Kalantari K. Hepatic hemangioma in a cluster of Iranian population. J med ultrasound. 2019;27:97.
- Imperiale A, Greget M, Chabrier G, Keomany J, Rust E, Detour J, Pessaux P, Goichot B. Solitary hepatic metastasis from medullary thyroid carcinoma mimicking atypical hemangioma: insights from multimodality diagnostic approach by MRI, F-18 FDG and F-18 FDOPA PET/CT. Clin Nucl Med. 2010; 35:434-437.
[Google Scholar] [CrossRef]
- Duxbury MS, Garden OJ. Giant haemangioma of the liver: observation or resection? Dig Surg. 2010; 27:7-11.
- Klotz T, Montoriol PF, Da Ines D, Petitcolin V, Joubert-Zakeyh J, et al. Hepatic haemangioma: common and uncommon imaging features. Diagn Interv Imaging. 2013;94:849-859.
- Memon Y, Malik NI, Anjum N, Ahmed SK, Saeed S. Ultrasound-guided Intralesional Bleomycin Injection (IBI) for treatment of cutaneous hemangiomas and vascular malformations. J Glob Radiol. 2016;2.
- Gupta VP, Gupta P. Intralesional bleomycin injection for periocular capillary hemangiomas. Indian J Ophthalmol. 2013; 61:367-378.
- McGahan JP, Roger E. Percutaneous sclerotherapy with bleomycin and ethiodized oil: a promising treatment in symptomatic giant liver hemangioma. Radiology. 2021; 301:464-471.
[CrossRef]
- Hashimoto M, Sugawara M, Ishiyama K, Sato T, Yamamoto Y, et al. Reduction in the size of a hepatic haemangioma after chemotherapy. Liver Int. 2008; 28:1043-1044.
- Nevesny F, Chevallier O, Falvo N, Guillen K, Malakhia A, et al. Bleomycin for percutaneous sclerotherapy of venous and lymphatic malformations: a retrospective study of safety, efficacy and mid-term outcomes in 26 patients. J Clin Med. 2021; 10:1302.
- Ketchum WA, Lin-Hurtubise KM, Ochmanek E, Ishihara K, Rice RD. Management of symptomatic hepatic “mega” hemangioma. Hawai'i J Med Public Health. 2019;78:128.
- Firouznia K, Ghanaati H, Alavian SM, Toosi MN, Daryani NE, et al. Management of liver hemangioma using trans-catheter arterial embolization. Hepat Mon. 2014;14.