Research Article - Onkologia i Radioterapia ( 2022) Volume 16, Issue 9
Phytochemical effects of soy isoflavones consumption on vitamin D and calcium levels in pre and postmenopausal women with hormone positive HER2 neu negative breast cancer
Ibtissam Abood Jabber1*, Asia Selman Abdullah1 and Haider Saadoon Qasim Alhilf22Department of Internal Medicine, College of Medicine, University of Misan, Misan, Iraq
Ibtissam Abood Jabber, Department of Pharmacology and Toxicology, College of Pharmacy, University of Basrah, Basrah, 61001, Iraq, Email: ahmedsalihdr2008@yahoo.com
Received: 31-Aug-2022, Manuscript No. OAR-22-73349; Accepted: 20-Sep-2022, Pre QC No. OAR-22-73349 (PQ); Editor assigned: 02-Sep-2022, Pre QC No. OAR-22-73349 (PQ); Reviewed: 16-Sep-2022, QC No. OAR-22-73349 (Q); Revised: 18-Sep-2022, Manuscript No. OAR-22-73349 (R); Published: 21-Sep-2022
Abstract
Breast cancer is the most common cancer in females, and it is ranked at level one in all cancer types list. Isoflavones are phenolic compounds with a chemical structure as same as estrogen which binds to hormone receptors. Calcitriol regulates proliferation, apoptosis, differentiation, inflammation, invasion, angiogenesis, and metastasis of BC. This study aimed to investigate the association of vitamin D and calcium levels with isoflavones in the premenopausal and postmenopausal women with hormone-positive Her2 neu negative BC treated with anti-estrogens and vitamin D. A randomized, interventional comparison study was carried out from October 2021 to May 2022. This study enrolled 120 BC, Iraqi women, with hormonal positive Her2 neu negative. The blood sample was collected in a gel tube and the serum is extracted as quickly as feasible. Then transferred into a new clean disposable plain tube to be utilized to assess the vitamin D3, and calcium for 3 months (at baseline, at a low dose, and a high dose of isoflavone). Their mean age was (47.66 ± 10.606) years with a median age of 46 years. The mean age of premenopausal women was (37.91 ± 3.476) years, while for postmenopausal females was (55.2 ± 8.12) years, with a highly significant difference (t=12.996; P<0.0001). There was a highly significant difference (t=3.175; P=0.003) between the BSA of post-menopause and pre-menopause women (1.78 ± 0.135 and 1.69 ± 0.143) Kg/m2, respectively. All postmenopausal women underwent surgical intervention (72, 100%), while thirty-nine premenopausal women were exposed to surgery, with a statistically significant difference (t=2.348; P=0.024).Four premenopausal women received Anastrazole (Arimidex) whereas 40(90.9%) received Tamoxifen, however, 62(86.1%) postmenopausal women received Arimidex, while the rest 10(13.9%) received Tamoxifen with highly statistically significant differences (t=-11.358-; P< 0.0001). According to oneway ANOVA analysis, the Ca2+ levels were raised significantly in both arms in particular during the administration of low and high doses of soya isoflavone (P<0.0001; 0.02). Moreover, the mean vitamin D concentrations at a low dose (35 mg) of soya isoflavone and a high dose (70 mg) of soya isoflavone in premenopause women were lower than in post-menopause women (23.48 ± 7.453 IU vs. 30.53 ± 10.981 IU; 26.7 ± 8.101 IU vs. 35.1 ± 9.395 IU), respectively. There was a high statistically significant difference (P <0.0001; P <0.0001), respectively. According to one-way ANOVA analysis, the vitamin D levels were raised significantly in both arms in particular during the administration of the low and high doses of soya isoflavone (P =0.046; <0.0001). Age, weight, comorbid, and positive family history of married women are significantly different between pre-menopause and post-menopause. Calcium shows different ranges, mostly non-significant after isoflavone consumption whereas vitamin D shows significant changes.
Keywords
breast cancer, vitamin D, calcium, isoflavone, soya
Introduction
Breast cancer (BC) is the most common cancer in females, and it is estimated that one in eight women in the US will develop BC in their lifetime [1]. In Iraq, the number of BC cases reached 4,542 in 2014 according to a WHO report [2]. In 2021, about 7,515 new cases of BC were recorded in Iraq, and 3,019 deaths were estimated [3]. Globally, an update published by GLOBOCAN found that female BC account for 2,261,419 new cases and 684,996 new deaths, it is ranked at level one in all cancer types list [4]. Soy-rich foods or isoflavones can reduce the risk of several chronic diseases [5-8], including certain forms of cancer, especially BC, and prostate adenocarcinoma [9-14]. The soybean, soybean, or soya bean is a species of legume, which is a type of natural isoflavonoids [15]. Clinically, the soy benefits have been studied with some evidence associated with decreased incidences of coronary heart disease, atherosclerosis, type II DM, and some cancer types. Several trials investigated soybeans as a potential agent for atrophy, menopause, and postmenopausal symptoms [16]. There are three soybean isoflavones which are genistein, daidzein, and glycitein. These non-steroidal compounds are naturally found in soybean and non-fermented soy foods primarily in their beta forms [17]. In the soybean, approximately genistin/genistein, daidzin/ daidzein, and glycitin/glycitein account for 55%, 45%, and 10% of total isoflavone content, respectively [18]. In terms of biochemistry, isoflavones are diphenolic compounds with a chemical structure as same as estrogen which binds to all ERα and ERβ [19, 20].
Vitamin D3 is the precursor to the potent steroid hormone calcitriol (1,25 dihydroxy vitamin D3 (1,25(OH)2 D3 )) that regulates the expression of many genes in most tissues of the body [21]. Dietary vitamin D3 is converted into 25 hydroxyvitamin D3 (25(OH)D3 ) in the liver, which is subsequently hydroxylase to form calcitriol by the cytochrome P450 enzyme CYP27B1 in the kidneys. Calcitriol regulates multiple signaling pathways involved in proliferation, apoptosis, differentiation, inflammation, invasion, angiogenesis, and metastasis of BC, and it, therefore, has the potential to affect cancer development and growth. Recent findings indicate that calcitriol also regulates microRNA expression and may affect cancer stem cell biology [21, 22].
This study aimed to investigate the association of vitamin D and calcium levels with isoflavones in premenopausal and postmenopausal women with hormone-positive HER2 neu negative BC treated with anti-estrogens and vitamin D.
Methods
Study design and setting
A randomized, interventional comparison study was carried out from October 2021 to May 2022. This study enrolled 120 BC, Iraqi women, with hormonal positive HER2 neu negative. A total of 72 women belonged to the postmenopausal group treated with anti-estrogen and the rest 48 women were in the premenopausal group.
Inclusion criteria
1. Patients should be diagnosed with BC hormonal positive ER+, PR+, and HER2 negative.
2. Patients start anti-estrogen treatment
3. The age is 25 years to 75 years.
Exclusion criteria
1. The age is under 25 years and older than 75 years.
2. Metastatic breast cancer.
Data Collection
A questionnaire will be filled out for each patient including the patient's characters and BC features, medical history, and frequency of soy food intake over the last year.
Blood sample
The blood sample w as collected in a gel tube and t he serum is extracted as quickly as feasible. For 10 minutes, the blood samples were centrifuged at 3000 rpm. Then transferred into a new clean disposable plain tube to be utilized to assess the Vitamin D3, and calcium for 3 months (at baseline, at a low dose, and a high dose of isoflavone).
Statistical analysis
Statistical analysis was performed using SPSS v24 (IBM Inc., Chicago, IL, USA). Descriptive statistics consist of numbers, and percentages were measured. Mean, median, and SD for categorical data were calculated. An association between premenopausal and postmenopausal was measured using an unpaired independent t-test. One-way ANOVA analysis was used to describe the association between groups. A two-sided P value of less than 0.05 was considered statistically significant.
Results
This s tudy e nrolled 1 20 B C Iraqi f emales; t heir m ean a ge was (47.66 ± 10.606) years with a median age of 46 years. Premenopausal women consisted of 48(40%), whereas postmenopausal females were 72(60%). The mean age of premenopausal women was (37.91 ± 3.476) years, while for postmenopausal females was (55.2 ± 8.12) years, with a highly significant difference (t=12.996; P<0.0001). The mean weight of premenopausal women (73.09 ± 12.421) kg was significantly lower than the weight of postmenopausal women (80.28 ± 17.063) kg, (t=2.058; P=0.046).
However, the overall mean and median weight was (76.27 ± 14.409; 75) kg. The mean height of premenopausal women (158.59 ± 7.167) cm was insignificantly lower than the height of postmenopausal women (159.61 ± 6.431) cm, (t=0.72; P=0.476). However, the overall mean and median height was (158.66 ± 6.767; 75) cm. As a result, the overall mean BSA was (1.74 ± 0.141) Kg/m2 with a median of (1.7) Kg/m2 . There was a highly significant difference (t=3.175; P=0.003) between the BSA of post-menopause and pre-menopause women (1.78 ± 0.135 and 1.69 ± 0.143) Kg/m2 , respectively. Regarding marital status, there was a high significant difference between premenopause (married=35) and post menopause (married=71) women (t=2.702; P=0.01). In relation to job, there was insignificant difference between pre-menopause (yes=9) and post menopause (yes=10) women (t=-1.159-; P=0.253). There was a highly statistical difference b etween p re-menopause (yes=8) and post-menopause (yes=51) related to comorbid conditions (t=6.312; P<0.0001), shown in (Table 1). According to past-surgical history, there was no relation between the two categories (t=0.476; P=0.636). Furthermore, there was no significant difference between pre-menopause (positive=18) and post-menopause (positive=22) women concerning family history, (t=-1.301-; P=0.2), (Table 1).
| Variables | Premenopausal women (n= 48)* | Postmenopausal women (n= 72) | t -test | P value | |
|---|---|---|---|---|---|
| Age (years) | 37.91 ± 3.476 | 55.2 ± 8.12 | 12.996 | <0.0001 | |
| Weight (Kg) | 73.09 ± 12.421 | 80.28 ± 17.063 | 2.058 | 0.046 | |
| Height (cm) | 158.59 ± 7.167 | 159.61 ± 6.431 | 0.72 | 0.476 | |
| BSA (m2) | 1.78 ± 0.135 | 1.69 ± 0.143 | 3.175 | 0.003 | |
| Married | Yes | 35 (79.5) | 71 (98.6) | 2.705 | 0.01 |
| No | 9 (20.5) | 1 (1.4) | |||
| Job | Yes | 9 (20.5) | 10 (13.9) | -1.159- | 0.253 |
| No | 35 (79.5) | 62 (86.1) | |||
| Co-morbid | Yes | 8 (18.2) | 51 (70.8) | 6.312 | <0.0001 |
| No | 36 (81.8) | 21 (29.2) | |||
| Past-surgical history | Yes | 47 (95.5) | 71 (98.6) | 0.476 | 0.636 |
| No | 2 (4.5) | 1 (1.4) | |||
| Family history | Yes | 18 (40.9) | 22 (30.6) | -1.301- | 0.2 |
| No | 26 (59.1) | 50 (69.4) | |||
Table 2 showed the comparison between pre-menopause and post-menopause women in this study about BC features. Staging (T (t=1.425; P=0.161) and N (t=0.198; P=0.84)), IHC (ER (t=1; P=0.323), PR (t=1; P=0.323) and HER2 neu (t=NA)), management (chemotherapy (t=-1.431-; P=0.16), and radiotherapy (t=-0.703-; P=0.486) had no significant differences among both arms. Regarding surgery, all postmenopausal women underwent surgical intervention (72, 100%), while thirty-nine premenopausal women were exposed to surgery, with a statistically significant difference (t=2.348; P=0.024).
Tab. 2. Breast cancer in this study (n=120)
| Variables | Premenopausal women (n= 48)* | Postmenopausal women (n= 72) | t -test | P value | |
|---|---|---|---|---|---|
| T | 2 | 25 (56.8) | 28 (38.9) | 1.425 | 0.161 |
| 3-4 | 19 (43.2) | 44 (61.1) | |||
| N | 1-2 | 31 (70.4) | 53 (73.6) | 0.198 | 0.84 |
| 3 | 13 (29.6) | 19 (26.4) | |||
| ER | Yes | 43 (97.7) | 72 (100) | 1 | 0.323 |
| No | 1 (2.3) | 0 | |||
| PR | Yes | 43 (97.7) | 72 (100) | 1 | 0.323 |
| No | 1 (2.3) | 0 | |||
| HER2 neu | Yes | 0 | 0 | NA | NA |
| No | 44 (100) | 72 (100) | |||
| Surgery | Yes | 39 (88.6) | 72 (100) | 2.348 | 0.024 |
| No | 5 (11.4) | 0 | |||
| Chemotherapy | Yes | 48 (100) | 68 (94.4) | -1.431- | 0.16 |
| No | 0 | 4 (5.6) | |||
| Radiotherapy | Yes | 41 (93.2) | 63 (87.5) | -0.703- | 0.486 |
| No | 3 (6.8) | 9 (12.5) | |||
| Types of hormonal therapy | Arimidex | 4 (9.1) | 62 (86.1) | -11.358- | <0.0001 |
| Tamoxifen | 40 (90.9) | 10 (13.9) | |||
*Four cases were missed of followup Regarding hormonal therapy, four (9.1%) premenopausal women received Anastrazole (Arimidex) whereas 40(90.9%) received Tamoxifen, however, 62(86.1%) postmenopausal women received Arimidex, while the rest 10(13.9%) received Tamoxifen with highly statistically significant differences (t=-11.358-; P<0.0001).
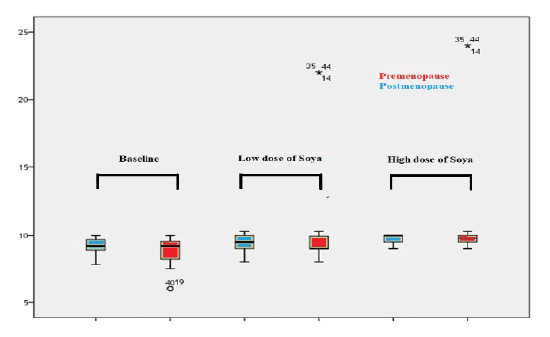
All biochemical results of this study included baseline concentration, the concentration at a low dose (35 mg) of soya isoflavone, and the concentration at a high dose (70 mg) of soya isoflavone were analyzed. Calcium (Ca2+) levels in post-menopause women and pre-menopause women were shown in Table 3.
Tab. 3. The comparison between mean levels of calcium of pre menopause and post menopause BC women
| Premenopausal women (n= 48) | Postmenopausal women (n= 72) | t -test (P value) | ||
|---|---|---|---|---|
| Ca2+ (mg/dL) | Baseline | 9.18 ± 1.685 | 9.2 ± 0.627 | 0.104 (0.918) |
| 1st | 10.1 ± 3.497 | 9.39 ± 0.577 | -1.45 | |
| 2nd | 10.78 ± 3.835 | 9.71 ± 0.371 | -1.859 | |
| ANOVA "F" (P value) | 235.05 (<0.0001) | 6.042 (0.02) | ||
t baseline, the overall mean Ca2+ level in pre-menopause women (9.18 ± 1.685mg/dL) was lower than in postmenopause women (9.2 ± 0.627mg/dL), with no significant difference (P=0.918). Moreover, the mean Ca2+ concentrations at low dose (35 mg) of soya isoflavone and at high dose (70 mg) of soya isoflavone in post-menopause women were lower than in pre-menopause women (9.39 ± 0.577mg/dL vs. 10.1 ± 3.497mg/dL; 9.71 ± 0.371mg/dL vs. 10.78 ± 3.835mg/ dL), respectively, in addition, there were no significant difference (P=0.23; P=0.084), respectively. According to one-way ANOVA analysis, the Ca2+ levels were raised significantly in both arms in particular during the administration of low and high doses of soya isoflavone (P<0.0001; 0.02), as shown in Figure 1.
Figure 1: . Box plot compared between mean levels of calcium of pre menopause and post menopause women
Vitamin D levels in post-menopause women and pre menopause women were shown in Table 4.
Tab. 4. The comparison between mean levels of vitamin D of pre menopause and post menopause women
| Premenopausal women (n= 48) | Postmenopausal women (n= 72) | t -test (P value) | ||
|---|---|---|---|---|
| Vitamin D | Baseline | 17.08 ± 6.569 | 20.743±8.133 | 2.53 (0.015) |
| (IU) | 1st | 23.48±7.453 | 30.53 ± 10.981 | 3.785 (<0.0001) |
| 2nd | 26.7 ± 8.101 | 35.1 ± 9.395 | 4.855 (<0.0001) | |
| ANOVA "F" (P value) | 3.648 (0.046) | 12.504 (<0.0001) | ||
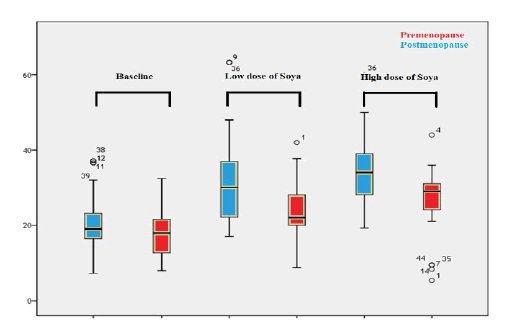
At baseline, the overall mean vitamin D level in pre-menopause women (17.08 ± 6.569 IU) was lower than in post-menopause women (20.743 ± 8.133 IU), with a statistically significant difference (P=0.015). Moreover, the mean vitamin D concentrations at a low dose (35 mg) of soya isoflavone) and at high dose (70 mg) of soya isoflavone in pre-menopause women were lower than in post-menopause women (23.48 ± 7.453 IU vs. 30.53 ± 10.981 IU; 26.7 ± 8.101 IU vs. 35.1 ± 9.395 IU), respectively. There was a high statistically significant difference (P<0.0001; P<0.0001), respectively. According to one-way ANOVA analysis, the vitamin D levels were raised significantly in both arms in particular during the administration of low and high doses of soya isoflavone (P =0.046; <0.0001), as shown in Figure 2.
Figure 2: Box plot compared between mean levels of vitamin D of pre menopause and post menopause women
Discussion
This study included 120 breast cancer Iraqi females; their mean age was (47.66 ± 10.606) years with a median age of 46 years. Premenopausal women consisted of 48(40%), the mean age of premenopausal women was (37.91 ± 3.476) years, whereas postmenopausal females were 72(60%), their mean age was (55.2 ± 8.12) years, with a highly significant difference (P<0.0001). These findings agreed with Al-Naqqash et al. Al-Alwan et al. and Al-Rawaq [28]. Yasui et al [29], recruited women whose ages were 45 to 54 years as 217(39.9%) premenopausal women (aged=47(44-49) years) and 327(60.1%) postmenopausal women (aged=52(50-53) years) [23-29]. Age is an important factor in the incidence and treatment of breast cancer [30]. In most Arabian regions, breast cancer is more commonly detected in women under the age of 50, unlike the Western countries, where women aged 50 years and older are most commonly diagnosed [31]. It has been proposed that these differences are due to changes in exposure to hormones, diet, physical activities, and other risk factors such as ethnicity, religion, and localities [32]. Younger generations are continuously detected with breast cancer, which has been comprehensively shown in the Iraqi Cancer Registry [33-37] and other documented reports from neighboring countries [38-40].
There w as a highly s ignificant di fference (P=0.003) bet ween the BSA of post-menopause and pre-menopause women (1.78 ± 0.135 and 1.69 ± 0.143) Kg/m2, respectively. These results are similar to data reported in previously Iraqi studies by AlNaqqash et al., Al-Alwan et al., and Al-Rawaq [23, 26, 28]. However, different studies estimated BMI rather than BSA [41, 42]. In a pooled analysis of prospective studies in breast cancer women, the authors demonstrated the risk of breast cancer to be 30% higher in women with a BMI over 31 m2/Kg compared with women with a BMI of 20 [41].
Regarding marital status, there was a highly significant difference between pre-menopause (married=35) and postmenopause (married=71) women (P=0.01). In total, 106 (88.3%) women in this study were married, while disagreed with the study of Alhelfi and Alhashimi, when reported 69.4% of females were married [43]. Concerning jobs, there was an insignificant difference between pre-menopause (yes=9) and post-menopause (yes=10) women (P=0.253). In general, 15.8% of women in the present study were employed, whereas the rest were jobless. Alhelfi and Alhashimi , who agrees with these, reported that 83.5% of women were housewives, and only 16.5% were an employer[43]. There was a highly statistical difference between pre-menopause (yes=8) and postmenopause (yes=51) related to co-morbid conditions (P<0.0001). This could be explained by the association of comorbid diseases with older age and hormonal changes. Furthermore, there was no significant difference between premenopause (positive=18) and post-menopause (positive=22) women concerning family history, (P=0.2). Approximately, 33.3% of women in this study had a positive family history of breast cancer; this is consistent with [43]. They mentioned that family history was documented in 32.9% of the sample study.
Many papers published by Al-Alwan et al., discussed breast cancer and its relation to family history, co-morbidities, medical and surgical history, and hormonal replacement therapy in Iraq, different percentages were obtained, respectively, with no significant differences [25-27]. These discrepancies between our study and other studies may be explained by there are no standard cancer registry programs, no accurate screening modalities, and may be related to socioeconomic and low educational levels.
The strongest predictors of distant metastasis, diseasefree survival, and overall survival of breast cancer are predictively influenced by tumor size (T stage), which correlates strongly with time to progression and prognosis [41, 42].
The lymph node status is the most important prognostic and risk factor and is directly correlated to survival and the best predictor of systemic micro-metastases in the future [42, 44]. In this study, seem to be all women were hormonal positive and HER2 neu negative, which explained by the selection process of inclusion and exclusion criteria of the study.
Regarding hormonal therapy, four (9.1%) premenopausal women received anastrozole (Arimidex) whereas 40(90.9%) received Tamoxifen, however, 62 (86.1%) postmenopausal women received Arimidex, while the rest 10 (13.9%) received Tamoxifen with highly statistically significant differences (P<0.0001). Cuzick et al., confirmed the long-term superiority and safety of anastrozole (Arimidex) over tamoxifen as initial adjuvant therapy for postmenopausal women with hormonepositive early breast cancer [45] . ATAC was the first trial that showed that an aromatase inhibitor is more effective and has fewer serious side effects than tamoxifen in the management of breast cancer in an adjuvant setting in postmenopausal women.
Overall, there was no difference in the occurrence of non-breast cancers, although there were some differences f o r p a rticular cancers. However, a causal relation for these differences is difficult to assess because of multiple comparisons [45].
Nowadays, EBCTCG mentioned that using an aromatase inhibitor rather than tamoxifen in premenopausal women receiving ovarian suppression will reduce the risk of breast cancer recurrence [46].
Lastly, the authors concluded for females with early-stage hormonal positive breast cancer, adjuvant treatment with five years of tamoxifen reduces their risk of death at 15 years by about one-third [47]. Aromatase inhibitors are an even more effective endocrine treatment than tamoxifen for postmenopausal women, with further proportional reductions in recurrence rates of about 30% [48].
At baseline, the overall mean Ca2+ level in pre-menopause women (9.18 ± 1.685) was lower than in post-menopause women (9.2 ± 0.627), with no significant difference (P=0.918). Moreover, the mean Ca2+ concentrations at a low dose and high doses of soya in post-menopause women were lower than in premenopause women (9.39 ± 0.577 vs. 10.1 ± 3.497; 9.71 ± 0.371 vs. 10.78 ± 3.835) mg/dL, respectively, with a non-significant difference. According to one-way ANOVA analysis, the Ca 2+ levels were raised significantly in both arms in particular during the administration of low and high doses of soya isoflavone (P<0.0001; 0.02). Steinberg et al., disagrees with the findings of this study, reported that calcium level non-significant changed in placebo group (9.5 ± 0.3; 9.5 ± 0.37; 9.5 ± 0.4) mg/dL, 80mg/d isoflavone (9.6 ± 0.3; 9.6 ± 0.36; 9.5 ± 0.4) mg/dL, and 120mg/d isoflavone (9.4 ± 0.3; 9.4 ± 0.36; 9.4 ± 0.4) mg/dL for baseline (P=0.86), one year (P=0.201) and two years (P=0.903) period in menopause women [49] .
Zhang et al. studied premenopausal Chinese women and found that mean changes from their corresponding baseline values of bone minerals density, calcium/phosphorus, vitamin D, and glutathione peroxidase activity were significantly increased, however, those of phosphorus, osteocalcin, luteinizing hormone and follicle-stimulating hormone were significantly dropped in isoflavone combined with calcium group [50]. The findings showed that soy isoflavone, calcium, and isoflavone combined with calcium therapy were effective and safe in attenuating BMD loss in premenopausal women, and isoflavone combined with calcium therapy was better than soy isoflavone and calcium alone.
There is, however, a paucity of information in the previous literature reporting on the clinical outcomes concerning the health benefits and potential risks of calcium depletion of soy isoflavone supplementation in postmenopausal women [49].
At starting baseline of this study, the overall mean vitamin D level in pre-menopause women was significantly lower than in post-menopause women, (P=0.015). Moreover, the mean vitamin D concentrations at a low dose (35 mg) of soya isoflavone and high dose (70 mg) of soya isoflavone in premenopause women were lower than in post-menopause women with a highly statistically significant difference (P<0.0001; P<0.0001), respectively.
According to one-way ANOVA analysis, the vitamin D levels were raised significantly in both arms in particular during the administration of the low and high doses of soya isoflavone (P=0.046;<0.0001). These discrepancies are supported by Marini et al, who reported BMD increases were greater with genistein for both femoral neck and lumbar spine compared to placebo, and genistein also significantly increased bone-specific alkaline phosphatase, vitamin D, and osteoprotegerin levels. In addition, genistein exhibited a promising safety profile with positive effects on bone formation in a cohort of osteopenic, postmenopausal women [51].
Wietzke and Welsh [52], found both phytoestrogens (resveratrol red wine and genistein soy) up-regulated the transcription of the VDR promoter gene in the breast cancer cell, as measured by reporter gene activity, approximately two-fold compared to vehicle-treated norm cells. Co-treatment with the anti-estrogen tamoxifen (TAM) in T47D cells and transfection in an estrogen receptor-negative breast cancer cell line demonstrated that the effects of phytoestrogens on the VDR promoter act dependently on the estrogen receptor. Resveratrol and genistein also increased VDR protein expression as detected by Western blotting methods [53]. Using resveratrol for treatment did not affect cell number or cell cycle profile while using genistein increased cell number. Because resveratrol could up-regulate VDR without increasing breast cancer cell growth [54].
Wietzke and Welsh, hypothesized that soy genistein mediated increase in VDR expression would sensitize breast cancer cells to the effects of 1,25-dihydroxy vitamin D3 and Vitamin D3 analogs [52]. These data support the concept that dietary factors, such as phytoestrogens, may impact breast cancer cell sensitivity to Vitamin D3 analogs through regulationag of the VDR promoter [55-58].
Wietrzyk et al., concluded isoflavonoids exert a regulatoryi function on the expression of cytochrome P450 enzymes and also up-regulate the vitamin D3 receptor (VDR) on breast cancer cells, which increases their sensitivity to 1,25-dihydroxyvitamin D3, the hormonally active form of vitamin D3 [54].
Also, isoflavonoids can raise the active form of vitamin D3 in serum due to their inhibitory activity on CYP24, the enzyme involved in the degradation of 1,25-dihydroxy vitamin D3 and its precursor 25-OH-D (3) to inactive compounds [56, 57].
Lechner and their colleagues, mentioned extra renal synthesis of the active vitamin D metabolite 1,25- dihydroxy vitamin-D3 (1,25-D) has been seen in cells derived from human organs prone to breast cancer incidence [59]. Enhancement of the synthesizing hydroxylase CYP27B1 and reduction of the catabolic CYP24 could support the local accumulation of the antimitotic steroid, thus preventing the formation of breast tumors.
Soya derivatives such as 17 beta-estradiol and genistein induced CYP27B1 but reduced CYP24 activity. These data indicate a potential, new approach for cancer prevention by counteraction of the 1,25-D-driven negative feedback, i.e., down-regulation of CYP27B1 and up-regulation of CYP24, which prevent its local accumulation with high susceptibility of mammary cells [54, 55].
The prevention of BC depends on the optimal synthesis of the antimitotic pro-differentiating vitamin D hormonal metabolite 1,25-(OH) (2)-cholecalciferol (1,25-D3). Authors suggested that nutritional soya especially genistein can optimize vitamin D3 synthesis, which could result in growth control of breast cancer cells and, conceivably, in inhibition of the progression of tumors [60-63]. Several studies were published in Basrah city about BC, otherwise, no one dealt with soya's effect on estrogens and vitamin D [64-66].
Conclusion
To the best of our knowledge, this is the first time study to determine the association of vitamin D and calcium levels with isoflavone intake in hormone-positive HER2 neu negative BC (pre and postmenopausal women) treated with nti-estrogens and vitamin D, particularly in Iraq, and enerally in Eastern Mediterranean countries. Calcium shows different ranges, mostly non-significant after soflavone consumption whereas vitamin D shows significant changes.
FUNDING SUPPORTING
None
Conflict Of Interest
None
Acknowledgement
Many thanks to Dr. Ahmed Salih Alshewered for his help.
References
- Jameel RF, Al-Naqqash MA, Al-Shawi NN, Al-Nuaimi HS. Cardiac Effect of Trastuzumab on Breast Cancer Women at Oncology Teaching Hospital/in Baghdad, Iraq.
- Wild C. World cancer report 2014. Wild CP, Stewart BW, editors. Geneva, Switzerland: World Health Organization; 2014.
- Cardiac Effect of Trastuzumab on Breast Cancer Women at Oncology Teaching Hospital/in Baghdad
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. 2021;71:209-249.
- Zhang X, Shu XO, Gao YT, Yang G, Li Q, et al. Soy food consumption is associated with lower risk of coronary heart disease in Chinese women. J Nutr. 2003;133:2874-2878.
- Kokubo Y, Iso H, Ishihara J, Okada K, Inoue M, et al. Association of dietary intake of soy, beans, and isoflavones with risk of cerebral and myocardial infarctions in Japanese populations: the Japan Public Health Center–based (JPHC) study cohort I. Circulation. 2007;116:2553-62.
[Google Scholar] [CrossRef]
- Ma DF, Qin LQ, Wang PY, Katoh R. Soy isoflavone intake increases bone mineral density in the spine of menopausal women: meta-analysis of randomized controlled trials. Clin Nutr. 2008;27:57-64.
- Liu J, Sun LL, He LP, Ling WH, Liu ZM, et al. Soy food consumption, cardiometabolic alterations and carotid intima-media thickness in Chinese adults. Nutr Metab Cardiovasc Dis. 2014;24:1097-1104.
- Atkinson C, Warren RM, Sala E, Dowsett M, Dunning AM, et al. Red clover-derived isoflavones and mammographic breast density: a double-blind, randomized, placebo-controlled trial [ISRCTN42940165]. Breast Cancer Res. 2004;6:1-0.
[Google Scholar] [CrossRef]
- Wu AH, Koh WP, Wang R, Lee HP, Yu MC. Soy intake and breast cancer risk in Singapore Chinese Health Study. Br J Cancer. 2008;99:196-200 .
- Wu AH, Wan P, Hankin J, Tseng CC, Yu MC, et al. Adolescent and adult soy intake and risk of breast cancer in Asian-Americans. Carcinogenesis. 2002;23:1491-1496.
- Hwang YW, Kim SY, Jee SH, Kim YN, Nam CM. Soy food consumption and risk of prostate cancer: a meta-analysis of observational studies. Nutr Cancer. 2009;61:598-606.
- Zhang M, Wang K, Chen L, Yin B, Song Y. Is phytoestrogen intake associated with decreased risk of prostate cancer? A systematic review of epidemiological studies based on 17,546 cases. Andrology. 2016;4:745-756.
- Applegate CC, Rowles III JL, Ranard KM, Jeon S, Erdman Jr JW. Soy consumption and the risk of prostate cancer: an updated systematic review and meta-analysis. Nutrients. 2018;10:40.
- Porcher MH. Multilingual multiscript plant name database. University of Melbourne www plantnames unimelb edu au/Sorting/Mushrooms_Intro html. 2005.
- Xiao CW. Health effects of soy protein and isoflavones in humans. J Nutr. 2008;138:1244S-1249S.
- Messina MJ, Wood CE. Soy isoflavones, estrogen therapy, and breast cancer risk: analysis and commentary. Nutr J. 2008;7:1-1.
- Yu L, Rios E, Castro L, Liu J, Yan Y, et al. Genistein: Dual Role in Women’s Health. Nutrients. 2021;13:3048.
- Kostelac D, Rechkemmer G, Briviba K. Phytoestrogens modulate binding response of estrogen receptors α and β to the estrogen response element. J Agric Food Chem. 2003;51:7632-7635.
- Zhang X, Wu C. In Silicon, In Vitro, and In Vivo Evaluation of the Developmental Toxicity, Estrogenic Activity, and Mutagenicity of Four Natural Phenolic Flavonoids at Low Exposure Levels. ACS omega. 2022;7:4757-4768.
- Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14:342-357.
- de La Puente-Yagüe M, Cuadrado-Cenzual MA, Ciudad-Cabañas MJ, Hernández-Cabria M, Collado-Yurrita L. Vitamin D: And its role in breast cancer. Kaohsiung J Med Sci.2018;34:423-427.
- Al-Naqqash MA, Al-Bdaer EK, Saleh WA, Al-Shewered AS. Progression free survival in Iraqi breast cancer patients treated with adjuvant 3D conformal radiotherapy: A cross-sectional study. F1000Research. 2019;8:71 .
- Al-Naqqash MA, Radhi SM, Kareem TF, Fawzi HA. Young age Iraqi women with breast cancer: an overview of the correlation among their clinical and pathological profile. Med Sci. 2019;23:6-11.
- Alwan NA, Kerr D, Al-Okati D, Pezella F, Tawfeeq FN. Comparative study on the clinicopathological profiles of breast cancer among Iraqi and British patients. Open Public Health J.2018;11.
- Jamal MY. Knowledge, screening, and practices surrounding Iraqi female breast cancer: an observational cross-sectional survey study. Prensa Med Argent. 2020;106:1 .
- Al-Naqqash M, Mohammed A, Albu-Sultan ZM, Hassan ES. The influence of Breast Cancer Molecular Subtypes on Metastatic pattern in Iraqi patients. Pak J Med Health Sci. 2020;14:863-873.
- Al-Rawaq KJ, Al-Naqqash MA, Jassim MK. Molecular Classification of Iraqi Breast Cancer Patients and Its Correlation with Patients’ Profile. J Fac Med Baghdad. 2016;58:197-201.
- Yasui T, Ideno Y, Onizuka Y, Nakajima-Shimada J, Lee JS, et al. The association of urinary estrogen levels with urinary isoflavone levels: Difference between premenopausal women and postmenopausal women. Maturitas. 2019;121:41-47.
- Sotiriou C, Neo SY, McShane LM, Korn EL, Long PM, et al. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci. 2003;100:10393-10398.
- National Cancer Institute. Surveillance, epidemiology, and end results (SEER) program. Cancer Stat Facts: Myeloma (2014–2018). 2018.
- Oussama MN. Guidelines for the early detection and screening of breast cancer. EMRO.
- Iraqi Cancer Board. Results of the Iraqi Cancer Registry. Baghdad, Iraqi Cancer Registry Center, Ministry of Health. 2009 .
- Iraqi Cancer Board. Results of the Iraqi Cancer Registry. Baghdad, Iraqi Cancer Registry Centre, Ministry of Health. 2004.
[Google Scholar] [CrossRef] - Iraqi Cancer Board. Results of the Iraqi Cancer Registry. Baghdad, Iraqi Cancer Registry Center, Ministry of Health. 2015 .
- Iraqi Cancer Board. Results of the Iraqi Cancer Registry. Baghdad, Iraqi Cancer Registry Center, Ministry of Health. 2018 .
- Iraqi Cancer Registry. Ministry Of Health, Iraqi Cancer Board, Baghdad.
- Alwan NA. Breast cancer: demographic characteristics and clinico-pathological presentation of patients in Iraq. EMHJ-East Mediterr Health J. 2010;16:1159-1164.
- Nageeti TH, Abdelhameed AA, Jastania RA, Felemban RM. Perspective of Saudi women in the Makkah region on breast cancer awareness. J Fam Community Med. 2017;24:97.
- El Shamaa ET, Hassanein MH. Risk factors of breast cancer in abha city: a case control study. Acad Res Int. 2012;2:34.
- Jameel RF, Al-Naqqash MA, Al-Shawi NN, Al-Nuaimi HS. Cardiac Effect of Trastuzumab on Breast Cancer Women at Oncology Teaching Hospital/in Baghdad, Iraq.
- Jameel RF, Al-Naqqash MA, Al-Shawi NN, Al-Nuaimi HS. Cardiac Effect of Trastuzumab on Breast Cancer Women at Oncology Teaching Hospital/in Baghdad, Iraq.
- Alhelfi HS, Alhashimi RA. Pattern of presentation of breast cancer in Missan's women. Int J Basic Appl Sci. 2015;4:162.
- Hansen EK, Roach M, editors. Handbook of evidence-based radiation oncology. N Y: Springer; 2010.
- Cuzick J, Sestak I, Baum M, Buzdar A, Howell A, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010;11:1135-1141.
- Bradley R, Braybrooke J, Gray R, Hills RK, Liu Z, et al. Aromatase inhibitors versus tamoxifen in premenopausal women with oestrogen receptor-positive early-stage breast cancer treated with ovarian suppression: a patient-level meta-analysis of 7030 women from four randomised trials. Lancet Oncol.2022;23:382-392.
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 2011; 378: 771–784.
[CrossRef]
- Early Breast Cancer Trialists' Collaborative Group. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386:1341-1352.
- Steinberg FM, Murray MJ, Lewis RD, Cramer MA, Amato P, et al. Clinical outcomes of a 2-y soy isoflavone supplementation in menopausal women. The American journal of clinical nutrition. 2011;93:356-367.
- Zhang X, Liu Y, Xu Q, Zhang Y, Liu L, et al. The effect of soy isoflavone combined with calcium on bone mineral density in perimenopausal Chinese women: A 6-month randomised double-blind placebo-controlled study. Int J Food Sci Nutr. 2020;71:473-481.
- Marini H, Bitto A, Altavilla D, Burnett BP, Polito F, et al. Breast safety and efficacy of genistein aglycone for postmenopausal bone loss: a follow-up study. J Clin Endocrinol Metab. 2008;93:4787-4796.
- Wietzke JA, Welsh J. Phytoestrogen regulation of a Vitamin D3 receptor promoter and 1, 25-dihydroxyvitamin D3 actions in human breast cancer cells. J Steroid Biochem Mol Biol. 2003;84:149-157.
- Wietzke JA, Ward EC, Schneider J, Welsh J. Regulation of the human vitamin D3 receptor promoter in breast cancer cells is mediated through Sp1 sites. Mol Cell Endocrinol. 2005;230:59-68.
- Wietrzyk J. Wpływ izoflawonoidów na aktywność przeciwnowotworową witaminy D3. Post Hig Med Dosw. 2007;61:253-260 .
- Danciu C, Avram S, Pavel IZ, Ghiulai R, Dehelean CA, et al. Main isoflavones found in dietary sources as natural anti-inflammatory agents. Curr Drug Targets. 2018;19:841-853.
- Gilad LA, Schwartz B. Association of estrogen receptor β with plasma-membrane caveola components: Implication in control of vitamin D receptor. J Mol Endocrinol.2007;38:603-618 .
- Gilad LA, Tirosh O, Schwartz B. Phytoestrogens regulate transcription and translation of vitamin D receptor in colon cancer cells. J Endocrinol. 2006;191:387-398.
- Gilad LA, Bresler T, Gnainsky J, Smirnoff P, Schwartz B. Regulation of vitamin D receptor expression via estrogen-induced activation of the ERK 1/2 signalling pathway in colon and breast cancer cells. J Endocrinol. 2005;185:577-592.
- Lechner D, Bajna E, Adlercreutz H, Cross HS. Genistein and 17β-estradiol, but not equol, regulate vitamin D synthesis in human colon and breast cancer cells. Anticancer Res. 2006;26:2597-2603.
- Yoo MY, Lee J, Chung JI, Yeo Y, Cho IY. The association between serum vitamin D concentration and colon polyp: a cross-sectional study using health care screening database in a tertiary hospital in Korea. Korean J Fam Med. 2021;42:303.
- Viggiani MT, Polimeno L, Di Leo A, Barone M. Phytoestrogens: Dietary intake, bioavailability, and protective mechanisms against colorectal neoproliferative lesions. Nutrients. 2019;11:1709.
- Kavoosi F, Dastjerdi MN, Valiani A, Esfandiari E, Sanaei M, et al. Genistein potentiates the effect of 17-beta estradiol on human hepatocellular carcinoma cell line. Adv Biomed Res. 2016;5.
- Abdulretha SD, Abdullah AS, AL‑Mozie’l MS. Phytochemical effects of genistein and daidzein on sex hormones and corticosterone in female adult rats exposed to Chlorpyrifos. Toxicol. Environ Health Sci.2022:1-7.
- Habib OS, Al-Imara KA. A changing pattern of cancer–related mortality in Basrah. Basrah J Surg. 2018;24:24-29.
- AA Al-Hilfi R, S Habib O. Cancer mortality in Basrah: A household survey results. Med J Basrah Univ.2015;33:10-16.
- Eady SJ, Ali HM, Al-Shawi AA. Preparation of novel Azo Dyes as a new anti-Human Breast Cancer MDA-MB231 Cells and study its association with DNA. J Basrah Res (Sci).2018;29:1-1.