Research Article - Onkologia i Radioterapia ( 2022) Volume 16, Issue 2
Preoperative cancer antigen 125 levels and its association with the stage and clinicopathological features of endometrial carcinoma, a retrospective study
Jehan M. Al-Musawi1* and Tlib H. Kamoona22Department of Haematology and Oncology, College of Medicine, Kufa university, Iraq
Jehan M. Al-Musawi, Department of Medical Oncology, College of Medicine, Kufa University, Iraq,
Received: 22-Feb-2022, Manuscript No. OAR-22-55146; , Pre QC No. P-55146; Editor assigned: 24-Feb-2022, Pre QC No. P-55146; Reviewed: 10-Mar-2022, QC No. Q-55146; Revised: 12-Mar-2022, Manuscript No. R-55146; Published: 15-Mar-2022
Abstract
Background: The role of preoperative cancer antigen 125 (CA-125) levels in predicting the behaviour of the tumour, its relation with the prognostic indicators of the patients and the optimal cut-off values is still controversial. Objective: This study aims to explore the role of preoperative CA-125 in predicting the prognostic parameters and aggressiveness of endometrial cancers and to define the best cut-off values. Patients and methods: It is a retrospective study involved the data of 53 eligible patients with endometrial cancer, who were admitted to the oncology teaching hospital in Baghdad during the period between January, 2018 and October, 2020. The patients’ clinical characteristics and tumour pathologic characteristics were compared with the preoperative CA-125 values using non parametric Mann-Whitney, Kruskal-Wallis, One-way ANOVA and Bonferroni post hoc analysis. The Receiver Operating Characteristic (ROC) curve was used to find the usefulness of the CA-125 test and the optimal cut-off values. Results: Preoperative CA-125 values showed a significant relationship with the pathologic characteristics of the tumour; including the grade, stage, histotype, lymphovascular space invasion and the extent of invasion, while they did not show a meaningful association with the clinical characteristics of the patients. Preoperative CA-125 test showed to be useful in predicting advanced disease with a level of 30.25 U/ml being the optimal cut-off value for deep myometrial invasion and extra-uterine disease with a sensitivity of 63.2% and 75%, respectively, and a specificity of 85.3% and 86.5%, respectively. On the other hand, the value of 44.6 U/ml was the best cut-off for lymph nodes metastasis with a sensitivity of 66.7% and a specificity of 85.4%. Conclusions: Elevated preoperative CA-125 showed to be helpful in predicting aggressive pathologic behaviour and extra-uterine disease in patients with apparently early stage tumours and it would help determining the extent of surgical staging and pattern of follow up in those patients.
Keywords
endometrial carcinoma, preoperative ca-125, cut-off value, extrauterine disease
Introduction
Endometrial Carcinoma (EC), which is more broadly known as carcinoma of the uterine corpus, is the sixth most common cause of cancer in women around the world and its incidence is expected to increase further in the next decade [1]. About three quarters of women diagnosed with the disease are postmenopausal, with a median age of 60 years [2]. The exact cause of EC remains undefined, but there are many risk factors that have been involved in the pathogenesis of the disease. Older age, higher body mass index, sedentary life style, infertility, excess exogenous estrogens, diabetes mellitus and insulin resistance, hypertension, chronic use of tamoxifen and genetic factors have been shown to increase the risk of EC; while multiparity, physical activity, smoking and metformin use have been shown to be protective against EC [3].
Endometrioid, serous, and clear-cell carcinomas account for 75%-80%, 5%-10%, and 1%-5%, of EC respectively [4]. Carcinoma sarcoma, also called malignant mixed Mullerian tumour, accounts for 4.3% of uterine corpus cancers and mainly occur in elder women. It is classified as dedifferentiated carcinomas of the endometrium rather than as sarcomas. Endometrioid carcinoma consist of a range of cancers from well to poorly differentiated neoplasms (i.e., low to high grade tumours), while serous carcinoma, clear cell carcinomas and carcinosarcoma are high grade by definition and they tend to show an aggressive clinical behaviour [5]. The primary adverse prognostic factors of recurrence in EC include older age of the patient, higher stage of the disease at diagnosis, deep myometrial invasion, lymph nodes involvement, aggressive histologic type, higher grade, and larger size, lower location of the tumour, negative hormone receptors status, Lymphovascular Space Invasion (LVSI) and positive peritoneal cytology [6].
Unfortunately, information about EC in Iraq is very limited. According to the annual report of Iraqi cancer registry 2018, about 728 new cases of uterine corpus cancers were diagnosed in Iraqi women with incidence rate of 2.90 per 100,000 female, making it the second most common gynaecologic malignancy and the tenth most common solid tumour among Iraqi women. Majority of the patients were postmenopausal with a mean age at diagnosis of about 60 years and most of them were diagnosed between 45 and 70 years of age [7]. Most of the patients present with early stage, low grade tumours with endometrioid histology being the most common histologic type in Iraqi women with endometrial cancers [8].
Cancer Antigen 125 (CA-125) is a surface antigen of that is expressed by the epithelial cells of many types of tumours. It is encoded by the mucin 16 (MUC16) gene in humans and can be detected in the serum using a murine monoclonal antibody known as OC125 [9]. MUC16 antigen has been shown to play multiple biological roles in the process of tumorogenesis. Those roles included immunoprotection of cancer cell cells from innate immune cells, triggering cell signalling and resulting in increased cancer cell proliferation, in addition to prometastatic roles of MUC16 allowing increased tumour mass and metastasis [10]. CA-125 was first described while screening for antibodies that are increased against the ovarian cancer cells. In 1984, Niloff stated that CA-125 levels tend to be raised in patients with recurrent and advanced EC [11]. Consequently, there have been many studies that evaluated CA-125 as a potential diagnostic and prognostic biomarker for EC. They suggested its role as a prognostic marker for preoperative evaluation of patients with EC due its prognostic rather than diagnostic characteristics. Many researchers investigated the prognostic role of CA-125 in EC and showed that high levels of preoperative CA-125 are indicative of higher risk of metastasis and disease recurrence and emphasized its role as an independent predictor of survival. As a result, they suggested that preoperative assessment of CA-125 should be incorporated into the initial preoperative evaluation of EC patients [12, 13].
Although there are many studies suggesting a cut-off value of 35 IU/L as the preoperative CA-125 level in determining prognostic factors and survival in EC, many different upper and lower cut-off values have been suggested and could be found in the literature. However; the exact preoperative serum CA-125 cut-off value in EC is still unclear and controversial issue [14].
Patients and Methods
The present study is a retrospective observational single-centre study that was conducted at the Oncology Teaching Hospital in Baghdad to analyse the clinical and pathological features of surgically staged patients with endometrial carcinoma by reviewing the patients’ medical records and try to find their relation with preoperative levels of CA-125 levels which are also collected from the patients’ records.
A total of 53 patients with a diagnosis of endometrial carcinoma, whose diagnosis made on the basis of histopathology after surgical staging by a gynecologic oncologist, were included the study and their data were collected and analysed.
The study involved reviewing the medical records of patients with endometrial carcinoma who were admitted to the oncology teaching hospital in Baghdad during the period between January, 2018 and October, 2020. From 215 records of patients with endometrial carcinoma, 53 patients fulfilled the required inclusion criteria and were included in the study.
Inclusion criteria included patients who had surgery as primary treatment, patients who have preoperative CA-125 measurement, patients with complete surgical staging, and patients with complete medical records.
Exclusion criteria included patients who had neoadjuvant chemotherapy or radiotherapy as primary treatment, patients who did not have preoperative CA-125 measurement, patients with history of endometriosis or history of another malignancy as it may be associated with false elevation of CA-125 levels, and patients with incomplete staging and those with incomplete records.
All the 53 patients had CA-125 done preoperatively, and the diagnosis was made by a specialized pathologist after a complete surgical staging done by gynecologic oncologist according to the recent International Federation of Gynecology and Obstetrics (FIGO) guidelines including Total Abdominal Hysterectomy and Bilateral Salpingo-Oophorectomy (TH/BSO), and pelvic lymphadenectomy. Total omentectomy and pelvic ± para-aortic lymphadenectomy was also done for high risk patients. Results of peritoneal cytology were not available for most of the patients, so it was excluded from the comparative study. The data collected included clinical characteristics of the patients including the age, menopausal state, parity, Body Mass Index (BMI) and medical history of hypertension, Diabetes Mellitus (DM), hypothyroidism and Coronary Artery Disease (CAD). Tumor characteristics were collected from surgical pathologic reports which included the stage, grade, histopathologic type, LVSI, depth of myometrial invasion, lower uterine segment involvement, cervical stromal involvement, parametrial involvement, adnexal involvement, lymph nodes status, omental involvement and distant metastasis. For comparative purposes, the study population were divided into two groups; early stage or uterine confined disease which includes stage I and II cancers, and advanced stage or extra-uterine disease which include stage III and IV disease. Preoperative CA-125 measurement for each patient was recorded and correlated with the previous variables. CA-125 was measured in our institutional laboratories by enzyme linked fluorescent assay method on patients’ serum or plasma using VIDAS CA-125 II test which combines a 2-step enzyme immunoassay sandwich method with a final fluorescent detection (ELFA) with a cut-off value of serum CA-125 being 35 U/ml.
Data were analysed using statistical package for the social sciences IBMSPSS (version 24) computer software program. Descriptive statistics presented as frequency tables, Continuous variables were expressed as mean ± Standard Deviation (SD) or median and range and categorical variables as numbers and percentages. Analytic statistics as non-parametric Mann-Whitney U test, Kruskal-Wallis H test, One-way ANOVA test and Bonferroni post hoc analysis were used to find association between CA-125 with demographic and clinicopathological parameters. ROC curves are used to compare the usefulness of tests confirmed by logistic regression analysis and assessment of the sensitivity and the specificity. Youden index was used to determine the best cut-off values with 95% confidence interval. A two-sided P value of below or equal to 0.05 was considered to be statistically significant.
The study was approved by the scientific committee in Iraqi board of medical specialties. Data from medical records were collected after agreement of health authority at Oncology teaching hospital in Baghdad. No informed consent was required to be taken from the patients because of the retrospective nature of the study.
Results
A total of 53 patients with endometrial Carcinoma enrolled in this study, the mean age ± SD were 59.9 ± 8.9 years, ranging between 38-80 years. With 41.5% of patients were between 60-69 years. 34% of patients were nulliparous, while low-parity (<4 births) and multiparity (≥ 4 births) accounted for 17% and 49% of the patients, respectively. 84.9% of patients were post-menopausal. The mean BMI ± SD was 33.3 ± 8.1 kg/m2, with 60.4% were obese (BMI ≥ 30 according to World Health Organization (WHO) classification) [15]. Regarding the medical history, 69.9% and 45% of the patients had history of hypertension and DM, respectively. While, history of hypothyroidism and CAD was positive in 17% and 13.2% of the cases respectively, as shown in Table 1.
Tab. 1. Distribution of the patients according to the clinical characteristics
| Variables |
Number |
Percentage |
|
|---|---|---|---|
| Age | <50 years | 7 | 13.20% |
| 50-59 years | 16 | 30.20% | |
| 60-69 years | 22 | 41.50% | |
| ≥70 years | 8 | 15.10% | |
| Parity | Nulliparous | 18 | 34% |
| Low parity | 9 | 17% | |
| High parity | 26 | 49% | |
| Menopausal state | Premenopausal | 8 | 15.10% |
| Post-menopausal | 45 | 84.90% | |
| BMI | Normal | 5 | 9.40% |
| Overweight | 16 | 30.20% | |
| Obese | 32 | 60.40% | |
| History of hypertension | Positive | 37 | 69.90% |
| Negative | 16 | 30.10% | |
| History of DM | Positive | 24 | 45.30% |
| Negative | 29 | 54.70% | |
| History of hypothyroidism | Positive | 9 | 17% |
| Negative | 44 | 83% | |
| History of CAD | Positive | 7 | 13.20% |
| Negative | 46 | 86.80% | |
Regarding pathologic characteristics, endometrioid carcinoma was the most common histopathologic type and accounted for 75.5% of the cases, 11.3% of patients had serous type, 7.5% of patients had carcinosarcoma and 5.7% of patients had clear cell carcinoma pathology. In addition, 41.5% of patients had grade III, 34% had grade II and 24.5% had grade I. Most of the patients (69.8%) had early stage (uterine confined disease) with 47.1% of the patients had stage IA EC, While advanced stage (extra-uterine disease) which included stage III and IV disease accounted for 30.2% of the cases. LVSI was positive in 35.8% of the cases, as shown in Table 2.
Tab. 2. Distribution of the patients according to pathological characteristics
| Pathological parameters | Number | Percentage | |
|---|---|---|---|
| Histology | Endometrioid | 40 | 75.50% |
| Clear cell | 3 | 5.70% | |
| Carcinosarcoma | 4 | 7.50% | |
| Serous type | 6 | 11.30% | |
| Grade | G 1 | 13 | 24.50% |
| G 2 | 18 | 34% | |
| G 3 | 22 | 41.50% | |
| Stage | IA | 25 | 47.10% |
| IB | 5 | 9.50% | |
| II | 7 | 13.20% | |
| IIIA | 2 | 3.80% | |
| IIIB | 1 | 1.90% | |
| IIIC | 6 | 11.30% | |
| IVB | 7 | 13.20% | |
| LVSI | Positive | 18 | 35.80% |
| Negative | 35 | 66% | |
Concerning the extent of the disease and the degree of invasion: 35.8% of patients had deep myometrial invasion (>50% depth of invasion) and 35.8% of patients had lower uterine segment involvement, cervical stroma was involved in 22.6% of the cases.
The level of CA-125 was found to be more than 35 U/ml in 30.18% (16) of patients and level was equal or below 35 U/ml in 69.81% (37) of patients with a mean of 61.16 ± 146.7 U/ml and a median level of 14.4 U/ml ranging between (5.4 U/ml-859 U/ml) in the entire sample.
Neither the clinical characteristics nor the medical history of the patients had shown any significant association with the level of Extra-uterine extension including parametrial involvement and adnexal involvement occurred in 11.3% and 17% of the cases, respectively. Lymph Nodes (LN) were affected by the disease in 22.6% of the cases, while the omentum was involved in 11.3% of the samples. Distant metastatic disease occurred in 13.2% of the studied patients, Table 3.
Tab. 3. Distribution of the patients according to the extent of invasion
| Pathological parameters
|
Number | Percentage | |
|---|---|---|---|
| Deep myometrial invasion
|
Positive | 19 | 35.80% |
| Lower uterine segment involvement | Positive | 18 | 35.80% |
| Negative | 35 | 66% | |
| Cervical involvement | Positive | 12 | 22.60% |
| Negative | 41 | 77.40% | |
| Parametrial involvement | Positive | 6 | 11.30% |
| Negative | 47 | 88.70% | |
| Adnexal involvement | Positive | 9 | 17.00% |
| Negative | 44 | 83.00% | |
| LN involvement | Positive | 12 | 22.60% |
| Negative | 41 | 77.40% | |
| Omental involvement | Positive | 6 | 11.30% |
| Negative | 47 | 88.70% | |
| Distant metastasis | Positive | 7 | 13.20% |
| Negative | 46 | 86.80% |
preoperative CA-125 (p-value>0.05), Table 4. The histopathologic prognostic factors showed a significant association with preoperative CA-125 levels including the grade (p=0.001), histologic type (p=0.013), LVSI (p<0.001), the stage (p<0.001) and lower uterine segment involvement (p= 0.008), as shown in Table 5.
Tab. 4. Relation of clinical characteristics with preoperative CA-125 level
| Variables | Mean ± SD CA-125 (U/ml)
|
Median CA-125 (U/ml) | p value | |
|---|---|---|---|---|
|
Age |
<60 years | 78.49 ± 185.90 | 13.7 | 0.836* |
| ≥60 years | 47.60 ± 109.37 | 15.4 | ||
| Parity | Nulliparous | 40.84 ± 80.14 | 17.2 | 0.631** |
| Low-parity | 121.81 ± 281.02 | 13.6 | ||
| High-parity | 53.92 ± 116.06 | 17.5 | ||
| Menopausal state | Premenopause | 60.90 ± 119.13 | 13.65 | 0.836* |
| Postmenopause | 61.03 ± 152.27 | 16.4 | ||
| BMI | Non-obese | 32.38 ± 38.03 | 16.4 | 0.935* |
| Obese | 79.79 ± 185.10 | 14.3 | ||
| Hypertension | Positive | 49.05 ± 109.54 | 13.6 | 0.931* |
| Negative | 88.67 ± 211.35 | 15.85 | ||
| DM | Positive | 33.12 ± 33.71 | 18 | 0.300* |
| Negative | 84.08 ± 194.51 | 13.2 | ||
| Hypothyroidism | Positive | 22.76 ± 20.53 | 17.2 | 0.600* |
| Negative | 68.83 ± 159.97 | 17 | ||
| CAD | Positive | 106.31 ± 218.00 | 23.2 | 0.227* |
| Negative | 54.11 ± 134.82 | 13.65 | ||
| *Mann-Whitney U test, **Kruskal-Wallis H test, significant ≤ 0.05. | ||||
Tab. 5. Relation of histopathologic characteristics with preoperative CA-125
| Variable | Mean ± SD CA-125 (U/ml) | Median CA-125 (U/ml) | p value | |
|---|---|---|---|---|
| Grade | G 1 | 13.13 ± 5.58 | 13.1 | 0.001** |
| G 2 | 48.00 ± 138.31 | 13.5 | ||
| G 3 | 99.94 ± 186.17 | 45 | ||
| Histology | Endometrioid | 45.09 ± 107.58 | 13.55 | 0.013* |
| Non-Endometrioid | 109.98 ± 228.57 | 43.8 | ||
| LVSI | Negative | 16.27 ± 12.90 | 13.1 | 0.000* |
| Positive | 148.00 ± 231.07 | 58.7 | ||
| Stage | Early | 20.20 ± 26.77 | 13.25 | 0.000* |
| Advanced | 155.36 ± 243.55 | 59.2 | ||
| Lower uterine segment involvement | Negative | 43.40 ± 113.03 | 13.5 | 0.008* |
| Positive | 95.25 ± 196.02 | 43.8 | ||
|
*Mann-Whitney U test, **Kruskal-Wallis H test, significant ≤0.05. |
||||
The association between the grade and preoperative CA-125 in early stage disease (stage I and II) was assessed using One-way ANOVA test with Bonferroni post hoc involvement (p=0.005), omental involvement (p=0.002), and distant metastasis (p< 0.001), all have shown a significant association with elevated preoperative CA-125 levels as shown in Table 6.
Tab. 6. Relation of the extent of invasion with preoperative CA-125
| Variable | Mean ± SD | Median CA-125 (U/ml) | p value | |
|---|---|---|---|---|
| CA-125 (U/ml) | ||||
| Deep myometrial invasion | Negative | 21.77 ± 27.43 | 13.2 | 0.003* |
| Positive | 131.21 ± 229.59 | 45 | ||
| Cervical involvement | Negative | 43.13 ± 104.92 | 13.6 | 0.022* |
| Positive | 122.08 ± 237.68 | 44.4 | ||
| Parametrial involvement | Negative | 44.35 ± 125.69 | 13.7 | 0.009* |
| Positive | 191.48 ± 235.67 | 77.6 | ||
| Adnexal involvement | Negative | 44.87 ± 129.77 | 13.95 | 0.030* |
| Positive | 139.88 ± 202.48 | 60.4 | ||
| LN involvement | Negative | 24.22 ± 28.99 | 13.6 | 0.005* |
| Positive | 186.69 ± 276.50 | 58.7 | ||
| Omental involvement | Negative | 27.83 ± 33.84 | 13.6 | 0.002* |
| Positive | 320.91 ± 349.26 | 209.45 | ||
| Distant metastasis | Negative | 25.13 ± 28.64 | 13.55 |
0.000* |
|
Positive |
296.78 ± 325.16 |
152 |
||
|
*Mann-Whitney U test, significant ≤0.05 |
||||
analysis revealed that the grade 3 patients were significantly differed from grade 1 (p= 0.026) and grade 2 (p=0.043) patients with a significant general p value between the groups (0.021), as shown in Table 7 and 8.
Tab. 7. Relation of the grade with preoperative CA-125 in early stage EC
|
Grade |
Number |
Mean±SD |
Median |
p value |
|---|---|---|---|---|
|
G 1 |
14 |
12.90 ± 5.43 |
12.7 |
0.021* |
|
G 2 |
16 |
15.89 ± 13.10 |
9.8 |
|
|
G 3 |
7 |
44.68 ± 54.16 |
21.6 |
|
|
*One-way ANOVA test, significant ≤ 0.05 |
||||
Tab. 8. Post hoc analysis of patients according to the grade
|
Grade |
Mean Difference |
Std. Error* |
p value** |
|
|---|---|---|---|---|
|
1 |
2 |
-2.98 |
9 |
1 |
|
3 |
-31.77 |
11.38 |
0.026 |
|
|
2 |
1 |
2.98 |
9 |
1 |
|
3 |
-28.79 |
11.14 |
0.043 |
|
|
3 |
1 |
31.77 |
11.38 |
0.026 |
|
2 |
28.79 |
11.14 |
0.043 |
|
|
* Standard error, ** significant ≤0.05 |
||||
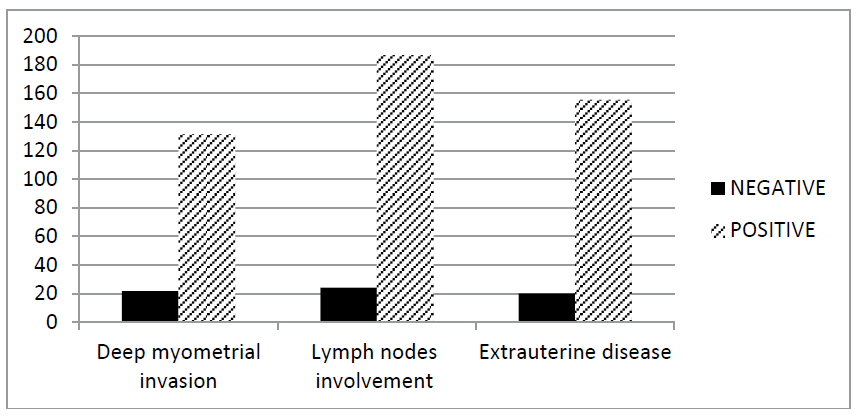
Figure 1 shows the distribution of the means of preoperative CA-125 values among the study patients according to three important prognostic pathologic parameters; depth of myometrial invasion, LN metastasis and the stage of the disease.
Figure 1: Distribution of CA-125 among the patients according to the depth of myometrial invasion, LN involvement and the stage of the disease
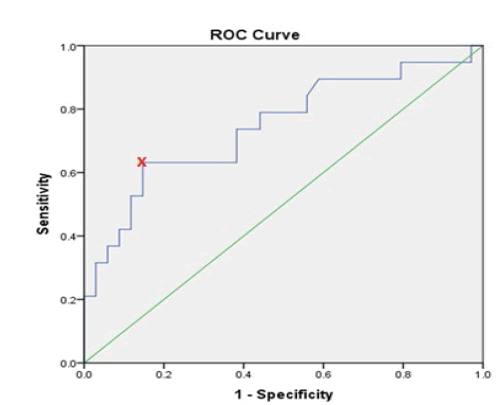
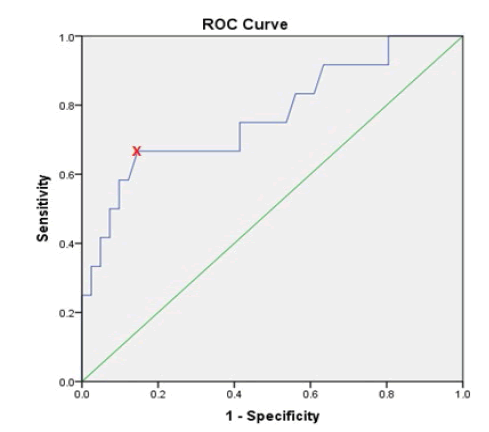
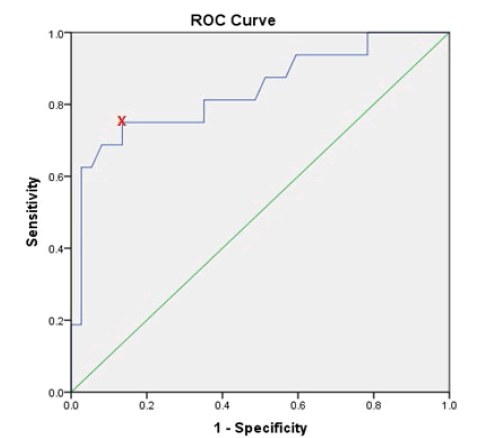
ROC curve and Youden index was used for determination of the optimal cut-off value of preoperative CA-125 values that help to predict deep myometrial invasion, lymph nodes involvement and extra-uterine disease. It showed that a preoperative CA-125 level of 30.25 U/ml is the optimal cut-off value in predicting deep myometrial invasion, which bears sensitivity, specificity, Positive Predictive Value (PPV) and Negative Predictive Value (NPV) of 63.2%, 85.3%, 70.6% and 80.5%, respectively. The cut-off value of 30.25 U /ml was also an optimal for predicting extra-uterine disease with a sensitivity, specificity, PPV and NPV of 75%, 86.5%, 70.6% and 88.8%, respectively. On the other hand, CA-125 level of 44.6 U/ml was the optimal cut-off point for prediction of lymph nodes metastasis with a sensitivity, specificity, PPV and NPV of 66.7%, 85.4%, 57.1% and 89.7%, respectively, as shown in Table 9.
Tab. 9. AUC values, sensitivity, and specificity of the optimal CA-125 cut-off values for poor prognostic pathologic parameters
| Prognostic variable | CA-125 cut-off value (U/ml) | AUC | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|
| Deep myometrial invasion | 30.25 | 0.745 | 63.20% | 85.30% | 70.60% | 80.50% |
| LN involvement | 44.6 | 0.769 | 66.70% | 85.40% | 57.10% | 89.70% |
| Extra-uterine disease | 30.25 | 0.837 | 75% | 86.50% | 70.60% | 88.80% |
Figures 2, 3 and 4 show the ROC curve for the association between preoperative CA-125 levels and deep myometrial invasion, LN involvement and extra-uterine disease with optimal cut-off values were determined according to Youden index.
Figure 2: ROC curve for deep myometrial invasion in relation to preoperative CA-125 levels
Figure 3: ROC curve for lymph nodes involvement in relation to preoperative CA-125 levels
Figure 4: ROC curve for extra-uterine disease in relation to preoperative CA- 125 levels
Discussion
EC is mainly a disease of elderly postmenopausal females, with endometrioid histology and stage I disease being the most commonly diagnosed histotype and stage among Iraqi women [8]. These facts have been supported by this study in which the mean age ± SD was 59.9 ± 8.9 years, with most of the patients being postmenopausal (84.9%). Those findings show agreement with the results of previous studies done by Yildiz et al, 2012 who found that the mean age was 60.19 ± 9.91years and 88.6% of the studies patients were postmenopausal [16]. Endometrioid histology and stage I disease accounted for 75.5% and 56.6% of the studied patients, respectively. Those results seem favorably comparable to a study by Modarres-Gilani et al, 2017, in which 79% and 62% of the patients had endometrioid histology and stage I disease, respectively [13].
The role of preoperative CA-125 in the diagnosis and management of endometrial cancer has not been clearly elucidated till now. In addition, different cut-off values have been suggested and explored by many researchers in an attempt to provide a predictive and prognostic tool that help to determine high risk patients preoperatively. In this study, 30.18% of the studied patients had elevated CA-125 (>35 U/ml) which appeared to be comparable with previous studies which reported CA-125 greater than 35 U/ml in 11-34.9% of patients with endometrial cancer [17].
Concerning clinical characteristics of the patients and their relation with preoperative CA-125 levels, the current study did not show any significant association between the age, menopausal state, parity, medical comorbidities or BMI of the patients with preoperative CA-125 values. When comparing these results with the previous studies, although Jiang et al, 2015 showed a significant association with the age and menopausal state of the patients [18], those results have not been proven in other studies by Knific et al, 2017, and Sebastianelli et al, 2010, who showed that the relation was non-significant between age, menopausal state, parity and medical history with pre-treatment CA-125 levels [19,20]. Although they did show a significant association with obesity, this relationship could not be proved by other studies done by Reijnen et al, 2019 and Wissing et al, 2019 failed to find any significant association [21,22]. Regarding to our study, it did not find a notable correlation between obesity and Ca-125 in Iraqi women.
The role of preoperative CA-125 as a prognostic marker and predictive of high risk histopathologic features has been confirmed in many studies. Goksedef et al, 2010 showed that high grade (grade 3), deep myometrial invasion (>50%), cervical stromal invasion, lymph nodes involvement and advanced stage were positively correlated with preoperative CA-125 values [23]. Angel Chao et al, 2013, Jiang et al, 2015, and Sebastianelli et al, 2010 agreed with those results and further showed a significant association of LVSI, adnexal involvement and distant metastasis and emphasized on the role of preoperative CA-125 level in predicting risk of recurrence and survival of patients with endometrial carcinoma [18-20]. These results come in parallel with the results of the current study and confirm the role of preoperative CA-125 in predicting high risk endometrial cancer patients in Iraq. In addition the results of post hoc analysis of grade 3 association with preoperative CA-125 values appear to be comparable to the results found by Yildiz et al, 2012 who found that the difference of grade 3 patients from grade 1 and grade 2 patients was statistically significant with p values of 0.034 and 0.044, respectively [16].
Regarding the histologic type, a meaningful association was found with preoperative CA-125 values in our study, although this association was not shown in the studies of Yildiz et al, 2012, Hapsari et al, 2019 and Abu Bakar et al, 2020 which were done on Turkish, south African and Malaysian patients, respectively [16,24,25]. These results may be explained that most of the study patients with non-endometrioid histology presented in advanced stage and no comparison could be done for early stage disease because of small sample size.
The statistically significant relationship between preoperative CA-125 values and poor prognostic histopathologic indicators of EC suggest that routine use of this test may of help to decide the need of more aggressive surgical staging and complete lymphadenectomy, especially for those with apparently early stage disease and those who prefer fertility preserving procedures. The association of preoperative CA-125 level with advanced stage endometrial cancer has been shown by many researchers, but the optimal cut-off value that has the best sensitivity and specificity in determining the extent of the disease still not clearly defined [26]. Yu-Li Chen et al, 2011 and Chin-Hsiung Hsieh et al, 2002 suggested that 40 U/ml would be a better cut-off value than 35 U/ml with a better sensitivity and specificity in predicting lymph nodes metastasis and the need for lymphadenectomy in EC patients [27, 28]. On the other hand, Modarres-Gilani et al, 2017, Jiang et al, 2015 and Nicklin et al, 2011 suggested lowering the cut-off value to 20, 25 and 30 U/ml respectively to predict advanced disease and worse survival in EC patients[13,18,29]. In a study done by Choi et al, 2005 who examined 42 patients, they found a statistically significant association between preoperative CA-125 levels and deep myometrial invasion, advanced stage, extra-uterine disease, and LN metastasis. They determined the optimal cut-off values for extra-uterine disease and LN metastasis to be 30 IU/ml and 50 IU/ml, respectively [30].
The findings of our study, and in agreement with the results of the studies mentioned above, showed that a CA-125 value of 30.25 U /ml was an optimal cut-off value in predicting deep myometrial invasion and extra-uterine disease. By using this cut-off value we would be able to detect 63.2% of the patients with deep myometrial invasion and 75% of the patients with extra-uterine disease preoperatively, with a specificity of 85.3% and 86.5%, respectively. On the other hand, CA-125 level of 44.6 U/ml was the optimal cut-off point for prediction of lymph nodes metastasis with a sensitivity and a specificity of 66.7% and 85.4%, respectively. Lowering the cut-off values would increase the sensitivity of the test on the expense of decreased specificity.
Limitations of the study included its retrospective nature, small sample size of this single-centre study, selection bias that could result from the exclusion of patients without preoperative CA-125 results and incomplete surgical staging. In addition, no comparison could be obtained regarding their progression-free and overall survival due to incomplete electronic data saving and lack of accurate survival information for most of the patients.
Conclusion
In summary, although limited number, the results of our study revealed that preoperative CA-125 testing has a significant association with the adverse prognostic parameters and pathologic predictors of aggressive disease. Routine use of preoperative CA-125 test may help to define high risk patients with apparently early stage disease who need more comprehensive staging studies, more aggressive surgical staging procedures and more frequent and careful follow up that could help to improve prognosis and survival of patients with endometrial cancer. Furthermore, the stratification of the cut-off values according to specific pathologic characteristics would improve the prognostic relevance of the test.
Aknowledgement
We, the authors would like to express our deep respect and sincere appreciation to all the gynaecologic oncologists at the gynaecologic oncology clinic in the Oncology Teaching Hospital in Baghdad for providing all the support, advice and cooperation needed to complete this study.
Competing Interest
The authors declare that they have no competing interests. The authors received no financial support for the research, authorship, and/or publication of this article.
References
- Ferrero S. Endometrial cancer: risk factors, management and prognosis. Nova Science Publishers. 2018.
Google Scholar Cross Ref - American Cancer Society. Endometrial Cancer. Am Cancer Soc. 2019.
Google Scholar Cross Ref - Raglan O, Kalliala I, Markozannes G, Cividini S, Gunter MJ, et al. Risk factors for endometrial cancer: an umbrella review of the literature. Int J Cancer. 2019;145:1719-1730.
Google Scholar Cross Ref - Murali R, Soslow RA, Weigelt B. Classification of endometrial carcinoma: More than two types. Lancet Oncol. 2014;15:e268-e78.
Google Scholar Cross Ref - Cantrell LA, Blank SV, Duska LR. Uterine carcinosarcoma: A review of the literature. Gynecol Oncol. 2015;137:581-588.
Google Scholar Cross Ref - Pineda MJ, Lurain JR. Uterine Corpus Cancer. In: The American Cancer Society, editors. The American Cancer Society's Oncology in Practice Clinical Management. 2018.
Google Scholar Cross Ref - Hussain A, Lafta RK. Cancer Trends in Iraq 2000-2016. Oman Med J. 2021;36:e219.
Google Scholar Cross Ref - Raham AM. The Clinicopathological Characteristics of Endometrial Carcinoma in Iraqi Women: Cross Sectional Study. Ind Public Health Develop. 2020;11:1789-1792.
Google Scholar Cross Ref - Bast RC, Badgwell D, Lu Z, Marquez R, Rosen D, et al. New tumor markers: CA125 and beyond. Int J Gynecol Cancer. 2005;15:274-281.
Google Scholar Cross Ref - Felder M, Kapur A, Gonzalez-Bosquet J, Horibata S, Heintz J, et al. MUC16 (CA125): Tumor biomarker to cancer therapy, a work in progress. Mol Cancer. 2014;13:1-15.
Google Scholar Cross Ref - Niloff JM, Klug TL, Schaetzl E, Zurawski VR, Knapp RC. Elevation of serum CA125 in carcinomas of the fallopian tube, endometrium, and endocervix. Am J Obstet Gynecol. 1984;148:1057-1058.
Google Scholar Cross Ref - Kwon JS. Preoperative CA-125 in low-grade endometrial cancer: Risk stratification and implications for treatment. J Gynecol Oncol. 2019;30(5):12–3. DOI: 10.3802/jgo.2019.30.e92.
Google Scholar Cross Ref - Modarres-gilani M, Vaezi M, Shariat M, Zamani N. The prognostic role of preoperative serum CA125 levels in patients with advanced endometrial carcinoma. Cancer biomarkers. 2017;20:135-141.
Google Scholar Cross Ref - Yilmaz Baran Å?, AlemdaroÄ?lu S, DoÄ?an DurdaÄ? G, Yüksel Å?imÅ?ek S, Aka Bolat F, et al. What is the predictive value of preoperative ca 125 level on the survival rate of type 1 endometrial cancer? Turkish J Med Sci. 2021;51:335-341.
Google Scholar Cross Ref - BMI classification. World Health Organization. 2021.
Google Scholar Cross Ref - Yildiz A, Yetimalar H, Kasap B, Aydin C, Tatar S, et al. Preoperative serum CA 125 level in the prediction of the stage of disease in endometrial carcinoma. Eur J Obstet Gynecol Reprod Biol. 2012;164:191-195.
Google Scholar Cross Ref - Chao A, Tang YH, Lai CH, Chang CJ, Chang SC, et al. Potential of an age-stratified CA125 cut-off value to improve the prognostic classification of patients with endometrial cancer. Gynecol Oncol. 2013;129:500-504.
Google Scholar Cross Ref - Jiang T, Huang L, Zhang S. Preoperative serum CA125: a useful marker for surgical management of endometrial cancer. BMC Cancer. 2015;15:396.
Google Scholar Cross Ref - Knific T, Osredkar J, Smrkolj Š, Tonin I, Vouk K, et al. Novel algorithm including CA-125, HE4 and body mass index in the diagnosis of endometrial cancer. Gynecol Oncol. 2017;147:126-132.
Google Scholar Cross Ref - Sebastianelli A, Renaud MC, Grégoire J, Roy M, Plante M. Preoperative CA 125 tumour marker in endometrial cancer: correlation with advanced stage disease. J Obstet Gynaecol Can. 2010;32:856-860.
Google Scholar Cross Ref - Reijnen C, Visser NCM, Kasius JC, Boll D, Geomini PM, et al. Improved preoperative risk stratification with CA-125 in low-grade endometrial cancer: A multicenter prospective cohort study. J Gynecol Oncol. 2019;30:1-11. DOI:.
Google Scholar Cross Ref - Wissing M, Mitric C, Amajoud Z, Abitbol J, Yasmeen A, et al. Risk factors for lymph nodes involvement in obese women with endometrial carcinomas. Gynecol Oncol. 2019;155:27-33.
Google Scholar Cross Ref - Goksedef BPC, Gorgen H, Baran SY, Api M, Cetin A. Preoperative Serum CA 125 Level as a Predictor for Metastasis and Survival in Endometrioid Endometrial Cancer. J Obstet Gynaecol Canada. 2011;33:844-850. DOI:.
Google Scholar Cross Ref - Hapsari K, Makin J, Dreyer G. The accuracy of preoperative serum CA-125 levels to predict lymph node metastasis in a population of South African women with endometrial carcinoma. South African J Gynaecol Oncol. 2019;11:7-10.
Google Scholar Cross Ref - Bakar NAA, Osman M, Hussin H. Preoperative serum CA 125 is associated with myometrial and cervical invasion in endometrial carcinoma. Malaysian J Med Heal Sci. 2020;16:75-81.
Google Scholar Cross Ref - Patsner B, Yim GW. Predictive value of preoperative serum CA-125 levels in patients with uterine cancer: The Asian experience 2000 to 2012. Obstet Gynecol Sci. 2013;56:281-288.
Google Scholar Cross Ref - Chen YL, Huang CY, Chien TY, Huang SH, Wu CJ, et al. Value of preoperative serum CA125 level for prediction of prognosis in patients with endometrial cancer. Aust N Z J Obstet Gynaecol. 2011;51:397-402.
Google Scholar Cross Ref - Hsieh CH, ChangChien CC, Lin H, Huang EY, Huang CC, et al. Can a preoperative CA 125 level be a criterion for full pelvic lymphadenectomy in surgical staging of endometrial cancer? Gynecol Oncol. 2002;86:28-33.
Google Scholar Cross Ref - Nicklin J, Janda M, Gebski V, Jobling T, Land R, et al. The utility of serum CA-125 in predicting extra-uterine disease in apparent early-stage endometrial cancer. Int J Cancer. 2012;131:885-890.
Google Scholar Cross Ref - Choi YS, Koh SB, Ahn JY, Yi CM, Shin IH, et al. Usefulness of preoperative CA 125 level in decision making of lymphadenectomy in endometrial cancer patients. Korean J Obstet Gynecol. 2005;48:2877-2887.
Google Scholar Cross Ref