Research Article - Onkologia i Radioterapia ( 2023) Volume 17, Issue 9
Prevalence of thyroid cancer among attendants of nuclear medicine department at oncology and nuclear medicine hospital in mosul city
Amina Nazar Abdulrazaq1*, Ammar H. Yahia1 and Ahmed Sabah Mohammed Jamee22Radiation Oncology Department, Oncology and Nuclear Medicine Hospital, Mosul, Iraq
Amina Nazar Abdulrazaq, Al-Quds Family Center, Nineveh Directory of Health, Mosul, Iraq, Email: dr.aminanazar@gmail.com
Received: 04-Sep-2023, Manuscript No. OAR-23-112605; Accepted: 02-Oct-2023, Pre QC No. OAR-23-112605 (PQ); Editor assigned: 06-Sep-2023, Pre QC No. OAR-23-112605 (PQ); Reviewed: 24-Sep-2023, QC No. OAR-23-112605 (Q); Revised: 30-Sep-2023, Manuscript No. OAR-23-112605 (R); Published: 06-Oct-2023
Abstract
Thyroid Cancer (TC) is the most commonly diagnosed endocrine cancer worldwide. There are an estimated 1,660 new thyroid cancer diagnoses and 237 thyroid cancer deaths in Iraq by the end of 2020. The study aimed to describe the prevalence of thyroid cancer among attendants of Nuclear Medicine department at Oncology and Nuclear Medicine hospital in Mosul City. A descriptive cross-sectional study design was conducted in the Nuclear Medicine Department at Oncology and Nuclear Medicine Hospital, Mosul, Iraq during six months from the first of January 2021 to the first of July 2021. A questionnaire form was specially prepared by the researcher, approved and modified by the supervisor, in order to collect all the relevant information related to the study. A total of 213 patients (175 females and 38 males) with mean age (41.6 ± 13.2) years diagnosed with thyroid cancer. The M: F ratio was 1:4.6. About 149(70%) were lived in urban regions, whereas 64(30%) were lived in rural areas. Only 7(3.3%) of patients had first degree and 6(2.8%) had second degree family history of thyroid diseases. The prevalence rate was 213/18000*100= 1.183%. Most of patients complained of neck lump or swelling in 134(62.9%). About 67/213 (31.5%) of patients presented with firm mass on examination. The mass rapidly increased in size in 25(11.7%) of patients. 44/213 (20.7%) of patients presented with palpable lymph nodes. About 71(33.3%) of patients shown marked hypoechogenicity on ultrasound, and 50(23.5%) were showed the presence of micro calcifications. Papillary TC was the most common type documented in 182(85.4%) of patients. The fourth and fifth decade of life are the prevalent age groups diagnosed with thyroid cancer? Females are still the predominant gender for thyroid cancer. The neck lump or swelling is considered as thyroid mass until proven otherwise. The rapidly increased in size firm mass with palpable lymph nodes is sign of thyroid cancer. The FNAB guide with neck ultrasound is empirical study to detecting thyroid cancer. Papillary TC is the most common type documented with localized disease.
Keywords
thyroid cancer, radioactive iodine, papillary TC, hurthle TC
Introduction
Thyroid cancers represent the most common endocrine neoplasms and comprise a spectrum of malignancies ranging from rarely lethal, slow-growing neoplasms to among the most aggressive cancers to afflict humanity [1, 2]. They usually present as anterior neck nodules, which in most patients can be located to the thyroid gland by palpation. Most masses are benign hyperplastic (or colloid) nodules, but 5% to 20% of nodules that come to medical attention are true neoplasms: benign follicular adenomas or carcinomas of follicular or para-follicular cell (C cell) origin. The differentiated thyroid cancers (those which are derived from follicular cells, including papillary, follicular, and Hurtle cell types) are typically associated with excellent survival and prognosis rates. In addition, differentiating true neoplasms from hyperplastic nodules and distinguishing benign from malignant tumours occasionally can be challenging. Highresolution ultrasonography studies assessing large groups of normal volunteers suggested that the prevalence of incidentally discovered nodular thyroid disease in healthy adults is more than 60% [3]. In the United States during 2018, the estimated new thyroid cancer cases were 53,990 (of these, 40,900 in women) [4]. In Iraq during 2020, the recorded new cases of thyroid cancer were 1,660(4.9%), which ranked in 5th position [5].
The goals of patient management are to minimize morbidity and mortality from cancer (tumour recurrence, metastases, and death) as well as from therapy (surgery, hypothyroidism, iodine-131 (131 I) therapy, Thyroid-Stimulating Hormone (TSH) suppression) while still achieving a favourable outcome. An early detection of nodular thyroid is an important advice to make seeking medical evaluation to differentiated nodule to benign or malignant. If the discovered lesion is suspected to be malignant, the patient can be advised that the management of typical thyroid cancer is effective and usually consist of surgical resection, followed by medical therapy and regular postoperative surveillance.
Globally, thyroid cancer was responsible for 586,202 (3%) new cases and 43,646 new deaths in the world in 2020 [6]. The incidence had tripled in the last four decades in the USA, since 1975 to 2009, the incidence increased from 4.9 cases to 14.3 cases per 100,000 individuals; with a more documented increase in women (from 6.5 to 21.4 cases per 100,000 women). It is ranked in the fifth position of the most common malignancy in women and the eleventh most common cancer overall. The global incidence rate in women of 10.1 per 100,000 is 3-fold higher than that in men, and the disease represents one in every 20 cancers diagnosed among women [6].
Mortality rates from the disease are much lower, with rates of 0.5 per 100,000 in women and 0.3 per 100,000 in men and an estimated 44,000 deaths in both sexes combined. Incidence rates are higher in transitioned countries than in transitioning countries, 4.0 times for men and 5.5 times for women. The highest incidence rates were found in Northern America, Australia/New Zealand, Eastern Asia, and Southern Europe for both sexes and also in Micronesia/ Polynesia, and South America for women. The highest global rates were estimated in Cyprus for both men and women [6-8]. In Iraq, the 5-years prevalence (all ages) was 11.93 per 100,000, and females were more detected than males (7.9% vs 2.5%), they were accounted 1,298 cases from 1660 cases of thyroid cancer in 2020.
Data from the National Cancer Institute’s (NCI) and Surveillance, Epidemiology, and End Results (SEER) program, demonstrate that the incidence of thyroid cancer steadily increased over the past three decades, increasing from a rate of 4.8 per 100,000 in 1975 to 15.0 per 100,000 in 2014, although more recently this rate has levelled off, with a rate ranging from 13.9 to 15.0 per 100,000 from 2009–2017 [9-10]. In the US, the Papillary Thyroid Carcinoma (PTC) accounts for approximately 80% of all thyroid carcinomas. Approximately 56,870 new cases of thyroid cancer and 2,010 disease-specific deaths occur each year in the US. It may occur at any age but is most commonly diagnosed in adults aged 45– 54 years old. Thyroid cancer death rates overall are <2% but vary significantly among the various types of thyroid cancer [10- 11].
The important prognostic factor in TC include age and gender of patients, histopathology, tumour size, tumour grade, autoimmune diseases, extra thyroidal invasion, nodal spreading, DM, secretion of thyroglobulin, calcitonin and carcinoembryonic antigen polypeptide markers, production of bFGF , EGFR , and VEGF, mutation of the TSH-R, aneuploid DNA, and RET/PTC rearrangements and RAS genetics alteration.
The survival for low-risk follicular or papillary carcinoma approaches is 100%, with long term follow-up. Although treated similarly to papillary thyroid cancer, invasive follicular carcinoma is more aggressive and carries a worse prognosis. Columnar and tall cell variants of papillary histology have a higher risk of recurrence. Hürthle cell carcinoma has worse prognosis than follicular histology, with twice the incidence of distant metastases. Anaplastic thyroid cancer patients usually survive<1 year despite aggressive therapy with a median survival of 6 months. Patients younger than 45 years of age with papillary or follicular carcinoma can have a surprisingly good outcome despite having systemic metastases. For medullary carcinomas <1 cm, Disease Free Survival (DFS) is 90%, but is only 50% for primary tumours >1 cm. The 5-years survival (%) by histology and stage as follow: Papillary: I (97%), II (93%), III (82%), IV (41%); Follicular: I (97%), II (89%), III (58%), IV (41%); Medullary: I (100%), II (88%), III (74%), IV (25%); and Anaplastic: IV (6%) [12].
The aims of the study are to describe the prevalence of thyroid cancer among attendants of Nuclear Medicine department at Oncology and Nuclear Medicine hospital in Mosul city
Methodology
Ethical and administrative considerations
The Medical Ethical Committee of Arab Board of Medical Specializations approved this study. An official agreement was obtained from the Ministry of Health and Directorate of Health in Mosul before conduction of the present study. A verbal consent was taken from all the participants in this study
Setting
The study was conducted in the Nuclear Medicine Department at Oncology and Nuclear Medicine Hospital, Mosul, Iraq. The hospital is located in left bank of Tigris River and it consists of three main departments (Oncology, Radiotherapy and Nuclear Medicine). The hospital which is staffed by specialists with the assistance of senior house officers delivers services to many areas in Mosul city and even to nearby provinces. The Nuclear Medicine Department had a filing system for each patient, each file contains a complete history about the patient who was examined by the specialist, then sent for investigations which determined the subsequent steps to be taken for the patient and even the follow up.
Study period
A descriptive cross-sectional study design was adopted in order to achieve the objectives of the present study.
Study sample and size
The sample included patients attending the Nuclear Medicine Department during the study period who had been diagnosed by an oncologist or nuclear medicine specialist to have thyroid cancer at Oncology and Nuclear Medicine Hospital.
Data collection
A questionnaire form was specially prepared by the researcher, approved and modified by the supervisor, in order to collect all the relevant information related to the study. The questionnaire form included information in regard to:
• The patients’ demographic data including: name, age, gender, marital status, and residency.
• Past history of thyroid disease (goitre).
• Past history of radiation exposure to the head and / or neck.
• Family history of thyroid disease / cancer.
The clinical details of the thyroid tumour were including: symptoms, signs, laboratory tests done (TSH, T3, T4), imaging studies performed used (neck ultrasound, thyroid scan, Fine Needle Aspiration Biopsy (FNAB), and thyroid lobectomy).
The variables related to histopathology of thyroid cancer were studied: type of cancer, and staging.
Follow-up was including: physical examination, Lab tests, and neck ultrasound.
Imaging performed on follow-up include: DXWBS, CXR, CT, MRI and PET scan.
This done for each patient within 1 month, 3 months, and 6 months. Through this period clinical examination and an ultrasound for neck was performed (to detect any remnant thyroid tissue, LAP, and secondary metastasis), then accordingly, other investigations done, which include: tumour markers, TSH, T3, T4, STg, Tg Ab, S. Ca+2 and Calcitonin. Also, DXWBS was done for detection of recurrence or relapse and metastasis. CXR, CT, MRI and PET scan for any recurrence or metastasis.
Statistical analysis
Study data were collected and processed using electronic data from the statistical analysis was performed using SPSS v24. Data were reported as means and standard deviations for ordinal variables or frequencies and percentages for categorical variables. The correlation coefficient of Pearson Chi-Square, and Fisher's exact test were used to evaluate the strength of the association. A P-value of less than 0.05 was considered statistically significant.
Results
Descriptive data analysis
Patient’s distribution according to marital status
A total of 213 patients (175 females and 38 males) with mean age (41.6 ± 13.2) years diagnosed with thyroid cancer were included and identified in this study. The mostly distributed age groups of patients were 31-40 and 41 years-50 years in 59(27.7%), and 57(26.8%), respectively. In relation to gender, males were (38, 17.8%), whereas females were (175, 82.2%), with no significant differences between groups (p-value=0.083). The M: F ratio was 1:4.6, as showed in (Table 1).
Tab. 1. Patient’s distribution according to age and gender
| Age (years) | Male | % | Female | % | Total | % |
|---|---|---|---|---|---|---|
| ≤20 | 0 | 0 | 4 | 1.9 | 4 | 1.9 |
| 21-30 | 5 | 2.3 | 31 | 14.6 | 36 | 16.9 |
| 31-40 | 8 | 3.8 | 51 | 23.9 | 59 | 27.7 |
| 41-50 | 12 | 5.6 | 45 | 21.1 | 57 | 26.8 |
| 51-60 | 4 | 1.9 | 30 | 14.1 | 34 | 16 |
| 61-70 | 7 | 3.3 | 9 | 4.2 | 16 | 7.5 |
| >70 | 2 | 0.9 | 5 | 2.3 | 7 | 3.3 |
| Total | 38 | 17.8 | 175 | 82.2 | 213 | 100 |
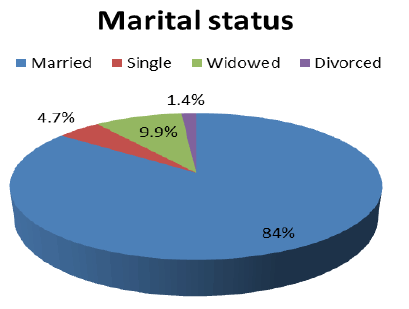
According to marital status, 179(84%) were married, 10(4.7%) were single, 21(9.9%) were widowed, and 3(1.4%) were divorced with a high significant differences between groups (p-value=0.0001), as showed in (Figure 1).
Figure 1:Patient’s distribution according to marital status
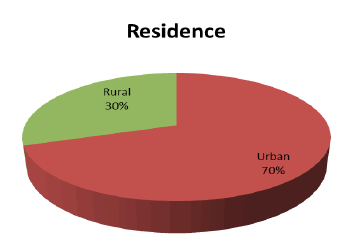
In relation to residence, 149(70%) were lived in urban regions, whereas 64(30%) were lived in rural areas without any significant difference (p-value=0.145), as showed in (Figure 2).
Figure 2:Patient’s distribution according to residence
Table 2 showed the distribution of patients according to past history of thyroid disease (goitre), past history of radiation exposure to head and or neck and family history of thyroid diseases/ cancer. Approximately, 95(44.6%) of patients had past history of goitre, while 118(55.4%) weren’t with no significant difference (p-value=0.371). The majority of patients 212(99.5%) hadn’t any history of radiation exposure with no significant difference (P-value=0.85). 200/213 (93.9%) of patients without family history of thyroid diseases or cancer. Only 7(3.3%) of patients had first degree and 6(2.8%) had second degree family history of thyroid diseases with a significant difference (p-value=0.023).
Tab. 2. Patient’s distribution according to past history
| Past history | No. | % | P-value | |
|---|---|---|---|---|
| Thyroid disease (goitre) | Yes | 95 | 44.6 | 0.371 |
| No | 118 | 55.4 | ||
| Radiation exposure to head and /or neck and | Yes | 1 | 0.5 | 0.85 |
| No | 212 | 99.5 | ||
| Family history of thyroid diseases / cancer | No | 200 | 93.9 | 0.023 |
| First degree | 7 | 3.3 | ||
| Second degree | 6 | 2.8 | ||
| Total | 213 | 100 | ||
Prevalence rate of thyroid cancer:
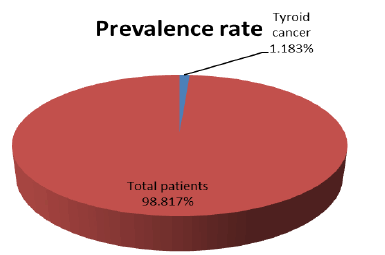
Totally, the patient’s attendances to hospital were 18000 during 6 months’ period of the study. The prevalence rate was 213/18000*100=1.183%, as showed in (Figure 3).
Figure 3: Prevalence rate of thyroid cancer
Thyroid cancer data analysis
Table showed the distribution of patients according to the symptoms and signs of thyroid cancer. Most of patients complained of neck lump or swelling in 134(62.9%). 13/213 (6.1%) felt of neck pain. About 22(10.3%) of patients complained of dysphagia. 10/213 (4.5%) of patients developed dyspnea. In addition, 33(15.5%) of patients suffered from more than one symptoms with no significant difference (p-value=0.5). About 67/213 (31.5%) of patients presented with firm mass on examination. The mass rapidly increased in size in 25(11.7%) of patients. 44/213 (20.7%) of patients presented with palpable lymph nodes. One patients felt of tenderness. In addition, 75(35.2%) of patients not showed any signs with no significant difference (p-value=0.09) (Table 3).
Tab. 3. Patient’s distribution according to the symptoms and signs of thyroid cancer
| Variables | No. | % | P-value | |
|---|---|---|---|---|
| Symptoms | Neck lump/swelling | 134 | 62.9 | 0.5 |
| Neck pain | 13 | 6.1 | ||
| HOV | 1 | 0.5 | ||
| Dysphagia | 22 | 10.3 | ||
| Dyspnea | 10 | 4.7 | ||
| More than one | 33 | 15.5 | ||
| Signs | Firm mass | 67 | 31.5 | 0.09 |
| Rapid increase in size | 25 | 11.7 | ||
| Tenderness | 1 | 0.5 | ||
| Palpable LN | 44 | 20.7 | ||
| Others | 1 | 0.5 | ||
| No signs | 75 | 35.2 | ||
| Total | 213 | 100 | ||
Imaging methods data analysis:
The results of neck ultrasound were shown in below mentioned table as followed: 71(33.3%) of patients shown marked hypo echogenicity, 13(6.1%) were showed taller than wide shape, 10(4.7%) were showed the presence of margin abnormalities, 50(23.5%) were showed the presence of micro calcifications, 8(3.8%) were showed aggressive growth (extension beyond capsule), 20(9.4%) were showed suspicious LAP, and 41(19.2%) were showed more than one feature in ultrasound with a high significant difference (p-value=0.019) (Table 4).
Tab. 4. Patient’s distribution according to neck ultrasound
| Ultrasound | No. | % | P -value |
|---|---|---|---|
| Marked hypoechogenicity | 71 | 33.3 | 0.019 |
| Taller than wide shape | 13 | 6.1 | |
| Presence of margin abnormalities | 10 | 4.7 | |
| Presence of microcalcifications | 50 | 23.5 | |
| Aggressive growth (extension beyond capsule) | 8 | 3.8 | |
| Suspicious LAP | 20 | 9.4 | |
| More than one | 41 | 19.2 | |
| Total | 213 | 100 |
Others investigation studies recorded in this study were thyroid scan in 3(1.4%), FNAB in 111(52.1%), and thyroid lobectomy in 47(22.1%) with no significant difference (p-value=0.06), as showed in (Table 5).
Tab. 5.Patient’s distribution according to the investigations performed
| Variables | No. | % | P- value |
|---|---|---|---|
| Thyroid scan | 3 | 1.4 | 0.06 |
| FNAB | 111 | 52.1 | |
| Thyroid lobectomy | 47 | 22.1 | |
| Others thyroidectomy | 3 | 1.4 | |
| Not done | 49 | 23 | |
| Total | 213 | 100 |
Histological data analysis:
Papillary TC was the most common type documented in 182(85.4%) of patients, followed by follicular TC in 20(9.4%), Hurthle cell carcinoma in 10(4.7%), and ATC in one patients with a high significant difference (p-value=0.004), as showed in (Table 6).
Tab. 6. Patient’s distribution according to the investigations performed
| Type | No. | % | P- value |
|---|---|---|---|
| Papillary | 182 | 85.4 | 0.004 |
| Follicular | 20 | 9.4 | |
| Hurthle cell | 10 | 4.7 | |
| Anaplastic | 1 | 0.5 | |
| Total | 213 | 100 |
Regarding stages of thyroid cancer, localized disease (I) found in 140(65.7%), local cervical invasion (II) recorded in 22(10.3%), lymph nodes positive (III) found in 46(21.6%), and DM found in 5(2.3%) with a strong significant difference (p-value<0.0001), as showed in (Table 7).
Tab. 7. Patient’s distribution according to the stages of thyroid cancer
| Stage | No. | % | P- value |
|---|---|---|---|
| Localized | 140 | 65.7 | <0.0001 |
| Local cervical invasion | 22 | 10.3 | |
| LN positive | 46 | 21.6 | |
| Distant metastasis | 5 | 2.3 | |
| Total | 213 | 100 |
Follow-up data analysis:
Approximately, 208/213 (97.7%) of patients completed the follow-up during this study, while only five (2.3%) patients lost to follow-up with no significant difference (p-value=0.95), as showed in (Table 8).
Tab. 8. Patient’s distribution according to the follow-up
| Follow-up | No. | % | P -value |
|---|---|---|---|
| Complete | 208 | 97.7 | 0.95 |
| Lost | 5 | 2.3 | |
| Total | 213 | 100 |
During follow-up, physical examination revealed neck swelling in 4.7% of patients and the same for lymph node enlargement with no significant difference (p-value=0.9), as showed in (Table 9).
Tab. 9. Patient’s distribution according to the physical examination on follow-up
| Physical examination | No. | % | P -value |
|---|---|---|---|
| Neck swelling | 10 | 4.7 | 0.9 |
| LN enlargement | 10 | 4.7 | |
| Normal | 193 | 90.6 | |
| Total | 213 | 100 |
Discussion
The results of this study revealed that the mostly distributed age groups of patients with thyroid cancer were 31 years-40 years and 41 years-50 years in 59(27.7%), and 57(26.8%), respectively with mean age was 41.6 years ± 13.2 years. These results were agreeing with that documented by Hamid, and Abdul Jabbar in Baghdad city. AL-Atrooshi studied 489 cases of thyroid cancer with age range between 15-68 years and median of 41.5 years [13-15].
A thesis introduced to College of Medicine/ University of Baghdad in 2017 about pattern of thyroid cancer in Iraq from 2000-2016 by Jabbar [15]. They assumed that thyroid cancer cases started to pick up by the thirties and continue to increase with advanced age. But his findings earlier than that reported in USA and UK which are in consistent with our results [17, 18].
Some authors find that age is related to PTC as a prognostic factor, while others don't, but it is not considered an independent predictor [19]. Even though certain authors consider age as an independent predictor for PTC for patients older than 45 years, but the autopsy studies do not show such pattern [20].
In relation to gender, females were predominant with MF ratio reached to 1:4.6. Most of previous studies recorded similar findings like Slijepcevic did not find sex to be an independent predictor for TC, while Noguchi S et al find a higher female to male ratio of TC in their study (9:1) [21, 22]. De Matos et al, agree with Slijepcevic, that sex is not an independent predictor for PTC, and explain the higher incidence of TC in women being the result of a higher incidence of benign thyroid disease in women [23].
The mean age at diagnosis of patients with PTC has been reported by different studies to be 41.9 years-48.5 years; it is much more common in female compared to males.
In this study, most of patients were married, lived in urban regions, half of them with history of goitre, nearly all patients hadn’t any history of radiation exposure. Low percent have family history of thyroid diseases. In Saudi Arabia, AL-Qahtani et al., found that the mean age at the time diagnosis of whole his cohort was 22.7 years (range: 5–30). The whole cohort (n=157) was consisted of 134 (85.4%) females and 23 (14.6%) males. Male gender was predominant in children (25.9%) than that in groups 19–25 years (10.3%) and 26–30 years (15.4%), respectively [24].
The prevalence of TC in this study was 1.183%. Most recently, the number of new cases of thyroid cancer is estimated to be 12.9 per 100,000 men and women annually, and the number of associated deaths is estimated to be 0.5 per 100,000 men and women annually. Still, the lifetime risk for thyroid cancer is approximately 1.1%, and the 5-year survival rate has risen to 97.8%, because almost 70% of cases are now diagnosed at an early stage, when the cancer is localized at the gland. The rise in the incidence of thyroid cancers may be attributable to the widespread use of imaging studies, such as ultrasounds, computed tomography, magnetic resonance imaging, and Positron Emission Tomography (PET) scans, that incidentally detect thyroid nodules [25].
Thyroid cancer occurs more frequently in women than in men, at an approximate ratio of 3:1, and is more prevalent in the white and Asian/Pacific Islander populations than in other populations. Thyroid cancer can occur in any age-group but more so in adults aged 45 years to 54 years, with a mean age of 50 years at diagnosis.
Most of patients complained of neck lump or swelling, and might be associated neck pain, dysphagia or dyspnea. The neck mass was firm on examination, rapidly increased in size accompanied with palpable lymph nodes. In term of clinical examination, the symptoms of thyroid cancer include a painless swelling in the front of the neck, difficulty swallowing, difficulty breathing, hoarseness, or a change in voice [26].
AL-Atrooshi and her colleagues showed data following clinical diagnosis (pre-operatively) of 362(74%) cases presented as goitre (multi-noduler enlargement of the thyroid gland), 28(5.7%) cases as toxic goitre, 63(12.9%) cases as solitary nodule, 9(1.8%) cases as tumor mass, 21(4.3%) cases as recurrent goitre, and 6(1.2%) cases presented as cystic lesions.
More than half of patients showed normal TSH, T3 and T4, whereas only several cases showed increased in TSH. The initial workup for any newly discovered thyroid nodule should include a serum Thyroid-Stimulating Hormone (TSH) level [27]. When thyroid hormone levels are low, the TSH rises responsively and vice versa; thus, measuring a TSH level allows differentiation between functional and non-functional nodules. The hyper functioning nodules are rarely malignant. However, if a TSH is subnormal, indicating a hyperactive gland, a nuclear medicine imaging study (thyroid uptake and scan) should be performed, to document whether the nodule itself is hyper functioning (hot), iso-functioning (warm), or non-functioning (cold) compared with the surrounding thyroid tissue [27].
The neck ultrasound is very important in this study which revealed 33.3% of patients shown marked hypo echogenicity, 6.1% showed taller than wide shape mass, 4.7% showed the presence of margin abnormalities, 23.5% were showed the presence of micro calcifications, 3.8% showed aggressive growth (extension beyond capsule), and 9.4% showed suspicious LAP. A diagnostic neck ultrasound should be performed on all suspected nodules to confirm the existence of a nodule and to check for any suspicious features. However, no single ultrasound feature and no combination of ultrasound features is sensitive enough or specific enough to identify malignancy by themselves [28].
Some ultrasound features have greater correlation with certain types of cancer, such as micro calcifications with papillary thyroid cancer and its absence in follicular thyroid cancer. Furthermore, certain sonographic features are highly predictive of benign nodules, such as purely cystic nodules and nodules with >50% spongiform appearance (aggregation of multiple micro cystic components) [28].
Musa et al., studied 79 cases (were predominately females; 67(84.8%) compared to 12(15.2%) males) with thyroid nodules, the mean age was 40.28 years ± 11.72 years. The initial assessments of clinical examination with assistance of ultrasound showed that the most of the patients had thyroid nodules in both lobes (53.2%), followed by 31.6% in right and 15.2% in the left lobe. In addition, the majority of patients had a multinodular goitre (65.8%) compared to 34.2% with solitary nodules [29].
Thyroid ultrasonography has been shown to be a useful tool to detect small nodules that surgeons unable to recognize on clinical examinations. It has been documented that the rate of thyroid nodules is between 30% and 50% by thyroid ultrasonography which 5-6.5% of them have malignancy [30].
There is no a single diagnostic method for definitive results of thyroid cancers, including ultrasound, radiography, scintigraphy, and suppression therapy to make a differentiation between benign and malignant lesions [30]. In this study thyroid scan done in 3 patients, FNAB in 105 patients, and thyroid lopectomy in 44 patients. A FNAB it remains the most accurate, cost-effective, and best diagnostic method for evaluating thyroid nodules [31]. The goal of the FNA biopsy is to obtain at least 6 follicular cell groups, each containing 10 to 15 cells from at least 2 different aspirates of a nodule for cytologic evaluation.
Generally, routine FNAB is not recommended for all nodules, unless their ultrasound appearance is suspicious. Yet, there are several contraindications include a history of high risk for malignancy, which include irradiation exposure, a family history of thyroid cancer, a previous hemithyroidectomy for thyroid cancer, or having positive nodules as determined by a PET scan.
Musa et al., found that sensitivity; specificity, positive predictive value, and a negative predicate value, and accuracy are 66.66%, 100%, 100%, 97.33%, and 97.50 respectively. These rations for FNAB in the literature are between 65% and 98% for sensitivity and between 73% and 100% for specificity [32-36].
Some studies have reported that diagnostic accuracy of FNAC for thyroid nodules can be improved with ultrasonography guidance resulting in lower false negative rates, in particular for impalpable lesions [37-39]. Namâ?Goong, reviewed retrospectively the medical records of the patients underwent ultrasound-guided FNAC and examined its role in thyroid malignant nodules. Subsequently, the sensitivity was reached to 90.9 for solid nodule and 68.2 for a hypo-echoic nodule [39]
Papillary TC was the predominant type documented in 85.4% of patients, followed by follicular TC. Localized thyroid cancer was common in 65.7%, followed by lymph nodes positive in 21.6% of cases. These results were almost always similar to all previously published literatures, recorded papillary carcinoma as high as 85% of malignant thyroid carcinoma which is the most common histo type of cancer.
Out of 213, 208(97.7%) were completed the follow-up during this study, while only five (2.3%) patients lost to follow-up. S.T.g should be measured every 6 to 12 months along with Tg Ab, because 25% of patients with thyroid cancer will produce these antibodies. Periodic neck ultrasound required in patients with partial thyroidectomy and in patients with total thyroidectomy who have not had 131I ablation to monitor for tissue growth [40-42].
A disease-free status is achieved when there is no clinical evidence of tumour, no imaging evidence of tumour (negative WBS), and undetectable STg level with TSH stimulation in the absence of Tg Ab. Such patients can then be followed up with annual thyroglobulin levels and thyroid hormone replacement.
In term of monitoring, at follow-up time when patient had high-risk disease, TSH should be suppressed to 0.1 to 0.5 mU/L for 5 to 10 years, and when patient have become disease free should maintain a TSH of 0.3 to 2.0 mU/L. When a patient with thyroglobulin positive (>10 ng/ mL) and the 131I WBS is negative, a PET scan should be requested to rule out any metastasis that would order further investigations. In persistent disease, TSH should be maintained to <0.1 mU/L indefinitely.
An increase in the prevalence of thyroid cancer has been observed for young and middle age groups due to the Hard conditions that passed through the Mosul city during the ISIS war and the use of lethal weapons, and globally banned chemicals in the last years before liberation, and all of this falls within the field of exposure and ocean radiation. Which is considered one of the most influential factors in the elevating rate of emergence and increase incidence of thyroid cancer.
Conclusion
The fourth and fifth decade of life are the prevalent age groups diagnosed with thyroid cancer. Females are still the predominant gender for thyroid cancer. There are no marking roles of marital status, residence, past history of thyroid disease, past history of radiation exposure to head and /or neck and family history of thyroid diseases/ cancer in the development of thyroid cancer. The neck lump or swelling is considered as thyroid mass until proven otherwise. The rapidly increased in size firm mass with palpable lymph nodes is sign of thyroid cancer. The FNAB guide with neck ultrasound is empirical study to detecting thyroid cancer. Papillary TC is the most common type documented with localised disease. The follow-up is the main stay in the monitoring of patients with thyroid cancer to evaluating patients after treatment modalities.
References
- Maxwell C, Sipos JA. Medical management of Thyroid Diseases: Differentiated thyroid carcinoma, Introduction. 3rd edt. NY, USA. Taylor Fr. Group LLC. 2019:181.
[CrossRef]
- Shindo H, Amino N, Ito Y, Kihara M, Kobayashi K et al. Papillary thyroid microcarcinoma might progress during pregnancy. Thyroid. 2014;24:840-844.
- Dean DS, Gharib H. Epidemiology of thyroid nodules. Best pract res Clin endocrinol metab. 2008;22:901-911.
- Siegel RL, Miller KD, Jemal A. Cancer statistics. CA: cancer j clin 2018;68:7-30.
- Al Alwan NA. Cancer control and oncology care in Iraq. J Contemp Med Sci. 2022;8:82-85.
- Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin med j. 2021;134:783-791.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: cancer j clin. 2018;68:394-424.
- Thuler LC. The Epidemiology of Stomach Cancer. Exon Publ. 2022:101-110.
- Medical management of Thyroid Diseases: Differentiated thyroid carcinoma, Introduction. 3rd edt. NY, USA
- SEER database. 2018.
- Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA otolaryngol–head neck surg. 2014;140:317-322.
- Wu WC, Schifftner TL, Henderson WG, Eaton CB, Poses RM, et al. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing noncardiac surgery. Jama. 2007;297:2481-2488.
- Hamid LS, Abdul Jabbar MQ, Sulaiman TI. Incidental Thyroid Carcinoma in Patients Treated Surgically for Thyroid Disease. J Fac Med Baghdad. 2019;61:60-67.
[CrossRef]
- Abdul Jabbar MQ, Mutlak NS, Abdul Hussein W, Sulaiman TI. Incidental thyroid carcinoma. J Fac Med Baghdad. 2016;85:245-249.
[CrossRef]
- Al-Atrooshi SA, Ibraheem NH, Yahya TT. The Prevalence of Papillary Thyroid Microcarcinoma in 489 Cases of Thyroidectomy in Iraqi Patients. Iraqi Postgrad Med. J. 2017;16:151-158.
- Jabbar SA. Pattern of Thyroid Cancer in Iraq from 2000-2016. Dissertation. 2017
[CrossRef]
- Papaleontiou M, Norton EC, Reyes-Gastelum D, Banerjee M, Haymart MR. Competing causes of death in older adults with thyroid cancer. Thyroid. 2021;31:1359-1365.
- Papillary thyroid microcarcinoma might progress during pregnancy
- Ito Y, Miyauchi A, Kihara M, Higashiyama T, Kobayashi K. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid. 2014;24:27-34.
- Zhang H, Zheng X, Liu J, Gao M, Qian B. Active surveillance as a management strategy for papillary thyroid microcarcinoma. Cancer Biol Med. 2020;17:543.
- Slijepcevic N, Zivaljevic V, Marinkovic J, Sipetic S, Diklic A, et al. Retrospective evaluation of the incidental finding of 403 papillary thyroid microcarcinomas in 2466 patients undergoing thyroid surgery for presumed benign thyroid disease. BMC cancer. 2015;15:1-8.
- Noguchi S, Yamashita H, Uchino S, Watanabe S. Papillary microcarcinoma. World j surg. 2008;747-753.
- Sabino de Matos P, Ferreira AP, Ward LS. Prevalence of papillary microcarcinoma of the thyroid in Brazilian autopsy and surgical series. Endocr Pathol. 2006;17:165-173.
- Cramer JD, Fu P, Harth KC, Margevicius S, Wilhelm SM. Analysis of the rising incidence of thyroid cancer using the Surveillance, Epidemiology and End Results national cancer data registry. Surgery. 2010;148:1147-1153.
- Al-Qahtani KH, Tunio MA, Al Asiri M, Aljohani NJ, Bayoumi Y, et al. Clinicopathological features and treatment outcomes of differentiated thyroid cancer in Saudi children and adults. J Otolaryngol-Head Neck Surg. 2015;44:1-6.
- Thyroid cancer. MedlinePlus. 2021
- Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association (ATA) guidelines taskforce on thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167-1214.
- Nguyen QT, Lee EJ, Huang MG, Park YI, Khullar A, et al. Diagnosis and treatment of patients with thyroid cancer. Am health drug benefits. 2015;8:30.
- BAHADDIN MM, ABDI MA, MUSA DH. A clinicopathological study of thyroid nodules. Duhok Med J. 2019;13.
- Yunus M, Ahmed Z. Significance of ultrasound features in predicting malignant solid thyroid nodules: need for fine-needle aspiration. JPMA J Pak Med Assoc. 2010;60:848-853.
- Nikiforov YE, Yip L, Nikiforova MN. New strategies in diagnosing cancer in thyroid nodules: impact of molecular markers. Clin cancer res. 2013;19:2283-2288.
- Pandey P, Dixit A, Mahajan NC. Fine-needle aspiration of the thyroid: A cytohistologic correlation with critical evaluation of discordant cases. Thyroid Res pract. 2012;9:32-39.
- Bagga PK, Mahajan NC. Fine needle aspiration cytology of thyroid swellings: How useful and accurate is it? Indian J cancer. 2010;47:437-442.
- Jo VY, Stelow EB, Dustin SM, Hanley KZ. Malignancy risk for fine-needle aspiration of thyroid lesions according to the Bethesda System for Reporting Thyroid Cytopathology. Am j clin pathol. 2010;134:450-456.
- Khalid AN, Quraishi SA, Hollenbeak CS, Stack Jr BC. Fine‐needle aspiration biopsy versus ultrasound‐guided fine‐needle aspiration biopsy: Cost‐effectiveness as a frontline diagnostic modality for solitary thyroid nodules. Head Neck: J Sci Spec Head Neck. 2008;30:1035-1039.
- Haberal AN, Toru S, Özen Ö, Arat Z, Bilezikçi B. Diagnostic pitfalls in the evaluation of fine needle aspiration cytology of the thyroid: correlation with histopathology in 260 cases. Cytopathology. 2009;20:103-108.
- Nam‐Goong IS, Kim HY, Gong G, Lee HK, Hong SJ, et al. Ultrasonography‐guided fine‐needle aspiration of thyroid incidentaloma: correlation with pathological findings. Clin endocrinol. 2004;60:21-28.
- Baier ND, Hahn PF, Gervais DA, Samir A, Halpern EF, et al. Fine-needle aspiration biopsy of thyroid nodules: experience in a cohort of 944 patients. Am J Roentgenol. 2009;193:1175-1179.
- Kim DL, Song KH, Kim SK. High prevalence of carcinoma in ultrasonography-guided fine needle aspiration cytology of thyroid nodules. Endocr j. 2008;55:135-142.
- Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1-33.
- Smallridge RC, Ain KB, Asa SL, Bible KC, Brierley JD, et al. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2012;22:1104-1139.
- Wells Jr SA, Asa SL, Dralle H, Elisei R, Evans DB, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma: the American Thyroid Association Guidelines Task Force on medullary thyroid carcinoma. Thyroid. 2015;25:567-610.