Research Article - Onkologia i Radioterapia ( 2021) Volume 15, Issue 10
Study of the guidelines and approaches of breast carcinoma management during the COVID-19 pandemic
Yousif Abdallah*Yousif Abdallah, Department of Radiological Science and Medical Imaging, College of Applied Medical Science, Majmaah University, 11952, Al-Majmaah, Saudi Arabia, Email: y.yousif@mu.edu.sa
Received: 26-Sep-2021 Accepted: 21-Oct-2021 Published: 25-Oct-2021
Abstract
Background: The pandemic COVID-19 has had a significant impact on cancer treatment. Several non-urgent hospital operations have been delayed reducing SARS-CoV-2 exposures, and patient visits to health centres have decreased. As a public health measure, careful consideration should be given to whether to prescribe immunosuppressive medications considering the current outbreak. Due to the delay of many breast surgeries, neoadjuvant systemic therapy is increasingly used for breast cancer.
Materials and Methods: In some cases, the augmented application of genetic tumour reporting may be necessary to guide neoadjuvant treatment options for core biopsy specimens. To successfully implement breast carcinoma management during a pandemic, health professionals require multidisciplinary teamwork. This can vary in terms of the different phases, tumour type, patient general condition and performance comorbidities and hospital resources. This paper describes how the COVID19 epidemic of the females' cancers program is handled in breast carcinoma.
Results: In this study, algorithms were used to guideline tumour biology and the severity of pandemic control options, which were then tested. They are primarily concerned with clinical oncology management choices, and they determine that the general therapy guidelines provided by nationwide organizations, such as the COVID-19 Pandemic Breast Cancer Consortium, have become operational throughout the pandemic.
Conclusion: The guidelines presented in this paper can be tailored to meet the needs and resources of other institutions, as well as the practices of oncology. To help control the spread of SARS-CoV-2 and SARS-CoV-2 breast cancer therapy, these guidelines will be critical.
Keywords
COVID-19, breast carcinoma, radiation, oncology
Introduction
Coronavirus, also known as COVID-19, is a respiratory disease that has impacted our way of life. Because of the unpredictability of that scenario and potential changes in cancer treatment, people with serious illnesses like breast cancer and their loved ones were extremely distressed. According to the Centres for Disorders Management and Avoidance, most people infected with COVID-19 are at low risk of developing serious illness. Those who have been diagnosed with cancer, particularly breast cancer, should be aware that they are more likely than the general population to develop serious complications from COVID-19. Now, it is unknown whether a history of cancer increases the risk of developing serious COVID-19 problems. Chemotherapy, targeted therapy, and immune therapy can all cause lung problems in women with breast cancer. Those who have compromised immune systems or respiratory problems are much more likely to suffer serious consequences if they become infected with the virus. Most immune systems recover within a few months of completing these treatments. In contrast, how long it takes for the immune system to recover can be different for different people. If a patient has had COVID-19 treatments in the past, the vulnerability to major COVID-19 issues is unknown. In 2020, the World Health Organization declared SARS-CoV-2 [1]. An emerging respiratory disease caused by a novel coronavirus infection, citing over 100,000 cases of 2019 corona viral disease (COVID-19) [2]. According to preliminary research, people with cancer are more likely to develop lifethreatening diseases, particularly if they receive systemic cancer treatment within 14 days of their COVID-19 diagnosis [3- 5]. This also increases the likelihood of intubating and killing patients on mechanical ventilation who have metastatic cancer. Due to the detection of SARS-CoV-2 in multiple cancer centres, cancer patients at those facilities are concerned about contracting the virus from their surroundings [6-8]. Numerous countries have implemented measures to safeguard vulnerable populations against COVID-19 outbreaks and to aid in the prevention of future outbreaks. Patients who do not require immediate medical attention now have more flexible treatment options, including waiting periods before non-emergency treatments, home-based therapies, and telemedicine [9,10]. While numerous cancer and non-cancer-specific organizations have issued treatment guidelines, numerous diseases and noncancer- specific organizations have not. While a portion of the COVID-19 dataset has peaked, occurrences continue to rise, which is unlikely to have an immediate and short-term effect on cancer treatment. The COVID-19 Pandemic Breast Cancer Consortium has released preliminary management recommendations for individuals diagnosed with breast cancer. These recommendations include the following: Surgery, radiation, and systemic therapeutic intervention are all critical when it comes to these types of principles. When making treatment decisions, it is critical to consider both the nature of the disease and the patient's objectives [11,12]. The breast cancer management method described in this article is based on the approach taken by many cancers treatment centres worldwide during the H1N1 pandemic. While treating breast cancer, it considers the variables and health risks associated with the SARS-CoV-2 outbreak. Utilizing existing data to improve breast cancer treatment throughout this time enables us to provide these validated phase and subtype-specific procedures developed by multidisciplinary teamwork.
The Non-Invasive Carcinoma (DCIS)
When no evidence of invasive cancer (or micro invasion) is found in the ductal carcinoma in situ, Ductal Carcinoma In Situ (DCIS) is surgically treated. Those who have done so include the following [13-15]. Radiation oncologists can treat more patients more quickly following a pandemic outbreak if they delay intervention on new DCIS-negative ERs until after the pandemic peak period. ENA is the favoured treatment option for patients with ER-positive DCIS who wish to consult with oncologists and endocrinologists via telemedicine [16-20]. Estrogen-Receptor Antagonists (ERAs) are recommended for postmenopausal women, while tamoxifen is recommended for premenopausal women [21]. Individuals who have previously undergone Breast Preservation Surgery (BCS) for DCIS and are RE-positive and capable of initiating ET may be able to postpone or avoid radiation [22-24]. Another option is to forego radiation therapy if the risk of illness is high (low to interim, 2.5 cm, operative margins 3 mm) [25].
Invasive Breast Carcinoma (Stages I-III)
Immediate implementation of a multidisciplinary evaluation is critical for the majority of people newly diagnosed with invasive breast cancer at stage I-III. A select group of newly diagnosed patients with invasive breast cancer is not suitable for systemic neo-adjuvant surgery, so the surgery is also offered for patients with initial invasive carcinoma stages who have completed the systemic neoadjuvant therapy. Due to the variability in surgical availability, Many healthcare institutions use COVID-19 criteria in conjunction with institutional and community resources to determine which patients are more surgically suitable. BCS is the procedure is recommended. Following COVID-19 assessment, expander or immediate implant reconstruction is provided. Consideration is given to risks and co-existing conditions. With limited surgical options, it may be necessary to proceed in stages, beginning with the initial and subsequent surgeries following the pandemic that the BCS has established for the pandemicaffected breast. If the requested reconstruction procedure cannot be performed immediately, neoadjuvant systemic therapy can be used to postpone mastectomy until a more favourable time presents itself. In negative breast cancer for those who are newly diagnosed in the early stages of the disease. Triple-Negative Breast Cancer (TNBC) Clinical T1N0 and node-negative node disease are both recommended to undergo initial surgery; however, if chemotherapy is available, then adjuvant chemotherapy is not required. Adjuvant chemotherapy is normally started following upfront surgery, in people with T1a-bN0 TNBC with a pathology condition as well as advanced pathological stages following upfront operations. Non-anthracycline-containing regimens are preferred, such as TC, in the cohort with lowgrade T1cN0 TNBC, which require fewer clinical visits. The use of adjuvant dose-dense doxorubicin/cyclophosphamide in the treatment of pathologic T2-4 and/or N1-3 TNBC, in conjunction with paclitaxel weekly or dose-dense paclitaxel, is recommended (AC-T) [26-29]. Initially, the surgeon should operate on both node-negative and node-positive disease; however, if adjuvant chemotherapy is available, the patient will not require additional treatment. Individuals with T1a-bN0 TNBC are frequently treated with additional chemotherapy following disease-modifying surgery. To minimize clinic visits for the T1cN0 TNBC cohort, a regimen is preferred that does not include either of the mechanisms mentioned previously, such as Transfusion Control (TC). Paclitaxel is administered weekly to patients with T2-4 and/or N1-3 TNBC pathology (AC-T) [30-35].
Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer
The COVID-19 Pandemic Breast Cancer Consortium recommends that early-stage breast cancer T1N0 HER2- positive be confirmed by pathological adjuvant (with associated risks of hospitalization and immunosuppression), the condition be diagnosed with a pathological focus of less than 3 cm (T2) and N0 (or LGCB) also is recommended that before relying on systemic therapy as an adjuvant. The adjuvant chemotherapy or HER2-specific therapy do not recommend for T1aN0 disease [36,37]. The advantages and disadvantages of HER2 adjuvant therapy are discussed in patients with HR-negative, Grade 3 conditions who have progressed to the T1bN0 pathological stage, as well as patients with HR-negative and/or Grade 3 conditions who are still in the T1bN0 pathological stage. Paclitaxel (or trastuzumab) plus emtansine (or T) is recommended for patients with pathologic T1bN0 disease who are considering adjuvant chemotherapy and HER2 treatment, whereas trastuzumab emtansine (or T) is recommended for patients with pathologic T1cN0 disease (or those with 3 cm small T2N0 disease) (TDM1). Despite their distinct toxicity profiles, the adjuvants T-H and T-DM1 have extremely high disease-free survival rates and cause minimal hematologic damage when compared to other adjuvants [38,39]. The adjuvant T-DM1 may be beneficial in the event of a pandemic in the near future. Additional therapy, such as routine HER2 adjuvant chemotherapy and Docetaxel/Carboplatin/Trastuzumab (TCH) therapy, should be recommended for patients who develop more severe illnesses following their initial surgery (P). Adjuvant systemic therapy for HER2-positive breast cancer is typically initiated 60 days after surgery, as is the standard of care. Targeted therapy should be used in many patients diagnosed with a solid tumour who have a tumour size of 3 cm or greater and/or a clinically positive axillary lymph node (s). The paclitaxel/trastuzumab/ pertuzumab agents are preferred, but de-escalation may be used in some cases if necessary [40-41]. NACT/HER2 targeted therapy should be completed within 4-6 weeks of the surgical procedure's commencement. In the event that the operation is not available for some reason, HER2-targeted (HP) cycles are recommended [32]. If disease is discovered following surgery, we treat it with standard T-DM1 post-neoadjuvants. If surgical eradication of the disease has not completely eradicated all traces of it, adjuvant HP is used to ensure complete eradication. The time interval between HER2 and HP treatments be reduced from 12 to 6 months and that the time interval between treatments be increased from 3 to 4 weeks to aid in pandemic prevention. While administering trastuzumab subcutaneously is an intriguing concept, it must be done under the supervision of a physician and in accordance with the terms of a health insurance policy. After primary therapy for early breast cancer (such as HER2-positive primary therapy), radiation oncology should be consulted in accordance with established protocols. Additionally, many healthcare institutions do not recommend delaying radiation in cases of HER2-positive braxal cancer, as we do in cases of TNBC, and we consider locoregional recurrence. Adjuvant ET is frequently given to patients with HR+ and HER2+ breast cancer who have completed their primary treatment to help prevent disease recurrence.
HR-Positive Breast Cancer
It can be challenging for newly diagnosed HR-positive breast cancer patients with stage I-III cancer to make a treatment decision because, in the event of a pandemic, a treatment strategy that deviates from conventional paradigms may be necessary. In the early stages of newly diagnosed HR-positive breast cancer patients, many healthcare institutions recommend that biological risk factors be considered when treatment decisions are made. Low-risk biological characteristics indicate a low likelihood of a reaction and/or a significant benefit from (neo) adjuvant chemotherapy, whereas high-risk biological characteristics indicate the opposite. Women are widely accepted to be at a high clinical risk prior to menopause (irrespective of other characteristics) [40,41].
Surgical intervention is recommended for patients with clinical stage T1-3N0, HR-positive breast cancer who have low-risk biological characteristics but sufficiently aggressive tumours to qualify as operational candidates for up-front surgery. If initial surgery is not possible, neoadjuvant ET can be used to postpone operations for six to twelve months. Breast cancer surgery is a possibility, and if we have an operating candidate for a patient in clinical stages T1-3N1 or HR-positive T4N0-1, the breast cancer cells will be removed that are ready for surgery preoperatively before performing the operation. Selecting on core biopsy samples is a viable option if surgery is not possible or desired. This is especially true when large tumours or nodal involvement are present, as well as when neoadjuvant therapy has the potential to significantly reduce the amount of breast and axillary treatment required. If the risk is low, many healthcare institutions prefer a 6-month course of neoadjuvant ET; if the risk is high, many healthcare institutions consider NACT. When treating HR-positive breast cancer that has high-risk biological characteristics and was recently diagnosed, many healthcare institutions take a slightly different approach. When it comes to T1-3N0-1 or T4N0 disease, many healthcare institutions prefer to perform surgery as soon as possible if the procedure is available and the patient is a good candidate. Neoadjuvant therapy and the use of genomic profiling to better understand the tumour’s biology and to guide treatment decisions between NACT and ET neoadjuvant may be used to treat low-risk tumour types in the event of a performable initial operation. Due to the fact that genetic profiling indicates that the individual is at a higher risk, we recommend that they use NACT instead. When we have a low-risk genomic profile, even in the presence of additional biological risk, many healthcare institutions tailor treatment to the patient's specific needs. Patients diagnosed with HRN2- 3 breast cancer routinely request neo-adjuvant systemic therapy, regardless of the likelihood of future cancer relapse. To increase the likelihood of a successful outcome for individuals with heightened biological characteristics, many healthcare institutions support the NACT approach and discuss its benefits and drawbacks for individuals with normal Biological Equivalents. AI appears to be a more effective treatment option than tamoxifen for postmenopausal women undergoing neoadjuvant hormonal therapy for breast cancer. Neoadjuvant hormone therapies are contraindicated in premenopausal women prior to the administration of aromatase inhibitors. Although Ovarian Tamoxifen Function Suppression (OFS) is the preferred treatment option in this case, the technique is supported by limited data and requires close monitoring throughout the treatment process. The normal timeframe for deferring surgery in patients with ET neoadjuvant is 6-12 months; however, patients with high biological potential undergo preoperative monitoring and surgery as soon as possible after diagnosis. Once a surgery has been delayed for more than six to twelve months, neoadjuvant ET must be continued until the surgery can be performed again. The preference for NACT over AC-T for disease located in the node is influenced by the presence of early HR-positive breast cancer. It is recommended that you complete your NACT surgery within 30 to 60 days of completing the cancer treatment. In the event that surgery is not possible within that time frame, the patient will receive neoadjuvant ET. The patient is hoped to be able to undergo surgery later. The introduction of genetic profiling of operational specimens to the extent is supported that it has not been done previously. As part of our standard of care, we can provide adjuvant TC or AC-T chemotherapy if necessary. Even though the desired outcome may be minor in a limited node-positive cancer, many healthcare institutions prefer to avoid chemotherapy due to the side effects. Due to the incomplete genomic profiling of biopsy specimens, we consider these patients to have received neoadjuvant ET followed by residual disease surgery. On the other hand, adjuvant chemotherapy is recommended when the patient is at high risk. The decision was made to delay adjuvant chemical therapy for HR-positive breast cancer for up to 90 days following surgery in order to prevent additional COVID-19 spread. However, given the pandemic's unpredictable course, we advise caution in delaying treatment. Radiation oncology is assigned to patients with early breast cancer who have undergone surgery with or without adjuvant chemotherapy. Radiation may be delayed for a period of time in patients with HR-positive breast cancer who are also receiving adjuvant therapy. Due to the low likelihood of local recurrence in women aged 65 to 70 with small N0 HR-positive brain cancer who have received BCS adjuvant ET; these patients should receive low-risk radiation following treatment. Adjuvant ET administration (which has already occurred) should be delayed until after the pandemic (for eligible patients only). For clients already receiving monthly OPS, we offer self-administration or regular clinical injections every three months to help reduce doctor visits.
Secondary Breast Carcinoma Treatment
In the case of metastatic breast cancer, the application of the COVID-19 Pandemic Breast Cancer Consortium recommendations" (MBC) is recommended. 15 Patients who are currently receiving early palliative systemic therapy with the expectation of improving outcomes should continue to receive it. However, due to the potential for future risks and benefits of treatment, the treatment should be carefully considered. When it comes to tumour genomes, the next generation looks for them using sequence sequencing. Progression-monitoring therapy is recommended for HER2-positive MBCs with a low disease burden and a long period of stability. It should be administered every three to six months on a three- to six-month cycle. The patients who are already receiving HP maintain a four-week treatment interval. If patients had also receiving other treatments every four weeks, it is critical to maintain a consistent dose throughout the course of treatment. Trastuzumab-Deruxtecan therapy for Her2-positive MBCs should be used cautiously due to the treatment's significant risk of pulmonary toxicity. MBCs and specific HR-positive drugs, such as ET, are frequently kept on the market. The risk of toxic drug contamination in newly diagnosed or progressing HR-positive MBCs must be assessed, and the risks and benefits of treating them with tackled ET medications, as well as those with older comorbidity, must be weighed against the benefits of doing so. The dose of Denosum A and Zoledronic acid were recommended to be delayed or increased until after the pandemic's peak. Patients at risk of developing skeletal complications or who exhibit hypercalcemia-related symptoms are always treated as soon as they become aware of the problem. While there is currently no evidence to support the use of oral bisphosphonates to treat bone metastases, this does not preclude their use in the future in the absence of additional evidence. Finally, delaying routine scans is recommended unless they are absolutely necessary, tracking tumour markers, and increasing the interval between tests in MBC patients (Figure 1).
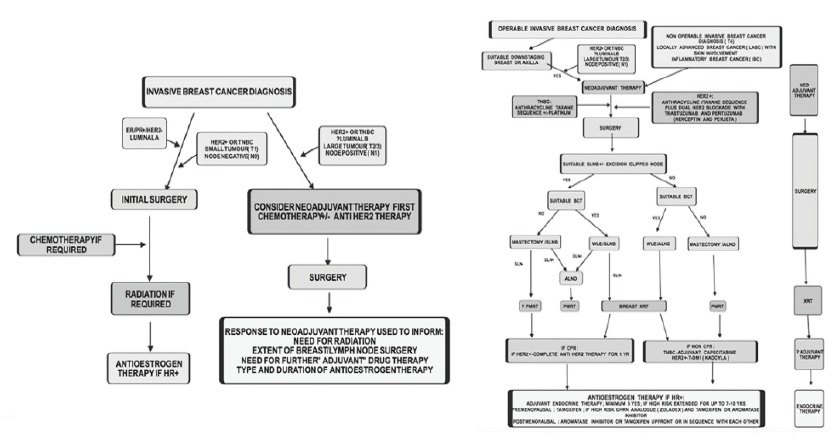
Figure 1: Algorithm of breast cancer treatment
Conclusion
Despite the fact that SARS-CoV-2 exposure poses a threat to both patients and health-care providers, it is recommended that both patients and health-care providers schedule appointments to detect oncological emergencies, such as tumour progression, recurrence or recurrence, new diagnoses, and unstable or symptomatic metastatic breast cancer. If patients are in the neoadjuvant stage, healthcare team is strongly advised patients to have a basic personal evaluation followed by additional inperson and telemedicine appointments. Healthcare teams want to examine the patient on a regular basis to assess the extent of the disease and the level of toxicity in the metastatic environment. The vast majority of other situations will necessitate patient's seeking telemedicine assistance. As a result, Healthcare teams did not have much time to put telemedicine in place in the weeks leading up to the outbreak of the virus. As state licensing regulations were relaxed, telemedicine became more user-friendly and cost-competitive, though it still required a steep learning curve for both patients and healthcare providers. A pandemic can be managed through the use of telemedicine, which can be used for routine visits to the doctor or postponed until after the crisis has passed. Although telemedicine and remote education can be used to manage the majority of ET side effects, such as hot flushes, joint pain, and sexual issues, many of these issues can also be prevented through the use of telemedicine and remote education. It is recommended that routine monitoring intervals (such as echocardiogram follow-up and ECG) be extended in order to delay adjuvant therapy and postpone it until after a possible pandemic. It is possible that the bone structure will be affected as well. Healthcare teams will continue to provide germ line testing for qualified candidates who request it, especially if the results have an impact on treatment options. A further goal is for young and eligible women who are considering systemic medications to remain sexually active prior to initiating treatment. It is recommended that treatment be changed in order to reduce immunosuppression and the need for frequent visits. When administered intravenously or orally, a higher frequency of administration and less immunosuppression should be used. Considering prophylactic growth factor in situations where it is not considered the norm may be appropriate in some situations. On chemotherapy days, we administer the medication through an on-body injector. Following that, we administer home growth factor administration. It is recommended that patient limit his/her use of steroids in order to help maintain his/her immune system. The Olanzapine-based emetic regimens have been developed for chemotherapy patients who experience moderate to severe nausea and vomiting. Once hypersensitivities have been avoided, dexamethasone is stopped, and once-weekly paclitaxel and intravenous dexamethasone are used instead of multiple oral doses, and docetaxel is administered intravenously instead of intramuscularly. The conclusion is that the risks of exposure and infections for COVID-19 are reduced as a result of the possibility of delays or less aggressive cancer treatment in resource-constrained environments. At this time, cancer screening is only available to patients who are asymptomatic. Testing can be viewed as an improvement in COVID-19 test capacity prior to the administration of chemotherapy. Healthcare teams are even more confident in the use of genome platforms for neoadjuvant therapy and remote care as a result of our breast cancer experience during the 2009 H1N1 pandemic, which has given us even more confidence. On the other hand, the outbreak has revealed systemic flaws such as economic inequality, a lack of health insurance, inadequately funded treatment, the incorrect use of biomarkers, and a lack of advance planning, among other things. This approach, while potentially useful for the selection and sequencing of future breast cancer medicines, is currently being used only in the COVID-19 pandemic to provide breast cancer care to those affected by the disease. In addition to global efforts to eliminate COVID-19, on-going national efforts to collect information on COVID-19 patients are a source of great excitement for us. In order to combat COVID-19, hospitals are concentrating their efforts, while the oncology community continues to provide high-quality care to patients affected by the pandemic's cancer burden.
Acknowledgement
Appreciation and thanks are due to Majmaah University for their research support.
References
- Bedford J, Enria D, Giesecke J, Heymann DL, Kobinger G, et al. COVID-19: towards controlling of a pandemic. Lancet. 2020;395:1015-1018.
- Chang D, Lin M, Wei L, Xie L, Zhu G, et al. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. JAMA. 2020;323:1092-1093.
- Coronavirus disease. 2019.
- Naming the coronavirus disease (COVID-19) and the virus that causes it. 2020.
- Sorbello M, El-Boghdadly K, Di Giacinto I, Cataldo R, Esposito C, et al. The Italian coronavirus disease 2019 outbreak: recommendations from clinical practice. Anaesthesia. 2020;75:724-732.
- Ueda M, Martins R, Hendrie PC, Crews JR, Wong TL, et al. Managing cancer care during the COVID-19 pandemic: agility and collaboration toward a common goal. J Natl Compr Cancer Netw. 2020;20:1-4.
- Coronavirus Update (Live): 3,335,283 Cases and 235,233 Deaths from COVID-19 Virus Pandemic-Worldometer.
- Liang W, Guan W, Chen R, Wang W, Li J, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335-337.
- Garg CC, Evans DB. World health organisation. What is the impact of non-communicable diseases on national health expenditure? 2011.
- Association of Breast Surgery. Statement from association of breast surgery. 2020.
- Fauci AS, Lane HC, Redfield RR. Covid-19-navigating the uncharted. N Engl J Med. 2020; 382:1268-1269.
- Yu J, Ouyang W, Chua MLK, Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020;6:1108-1110.
- Bartoszko JJ, Farooqi MAM, Alhazzani W, Loeb M. Medical masks vs N95 respirators for preventing COVID-19 in health care workers: a systematic review and meta-analysis of randomized trials. 2020. Influenza and other respiratory viruses. 2020;14:365-373.
- BASO-The Association for Cancer Surgery. Pragmatic management of breast cancer during COVID-19. 2020.
- Curigliano G, Cardoso MJ, Poortmans P, Gentilini O, Pravettoni G, et al. Recommendations for triage, prioritization and treatment of breast cancer patients during the COVID-19 pandemic. Breast. 2020;52:8-16.
- ARHAI Scotland. Rapid Review of the literature: Assessing the infection prevention and control measures for the prevention and management of COVID-19 in health and care settings. 2021.
- Extance A. Covid-19 and long term conditions: what if you have cancer, diabetes, or chronic kidney disease? BMJ. 2020;368:m1174.
- Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775-1776.
- Fan L, Liu S. CT and COVID-19: Chinese experience and recommendations concerning detection, staging and follow-up. Eur Radiol. 2020;6:1-3.
- World Health Organisation. Rational use of personal protective equipment (PPE) for coronavirus disease (COVID-19). 2020.
- Al-Balas M, Al-Balas HI, Al-Balas H. Surgery during the COVID-19 pandemic: a comprehensive overview and perioperative care. Am J Surg. 2020;219:903-906.
- Chagpar AB, Killelea BK, Tsangaris TN, Butler M, Stavris K, et al. A randomized, controlled trial of cavity shave margins in breast cancer. N Engl J Med. 2015;373:503-510.
- Marla S, Stallard S. Systematic review of day surgery for breast cancer. Int J Surg. 2009;7:318-323.
- Gallagher M, Jones DJ, Bell‐Syer SV. Prophylactic antibiotics to prevent surgical site infection after breast cancer surgery. Cochrane Database Syst Rev. 2019.
- Robertson JF, Todd JH, Ellis IO, Elston CW, Blamey RW. Comparison of mastectomy with tamoxifen for treating elderly patients with operable breast cancer. Br Med J. 1988;297:511-514.
- Mustacchi G, Scanni A, Capasso I, Farris A, Pluchinotta A, et al. Update of the Phase III trial ‘GRETA’of surgery and tamoxifen versus tamoxifen alone for early breast cancer in elderly women. Future Oncol. 2015;11:933-941.
- Spring LM, Gupta A, Reynolds KL, Gadd MA, Ellisen LW, et al. Neoadjuvant endocrine therapy for estrogen receptor-positive breast cancer: a systematic review and meta-analysis. JAMA oncol. 2016;2:1477-1486.
- Eiermann W, Paepke S, Appfelstaedt J, Llombart-Cussac A, Eremin J, et al. Letrozole neo-adjuvant breast cancer study group: preoperative treatment of postmenopausal breast cancer patients with letrozole: a randomized double-blind multicenter study. Ann Oncol. 2001;12:1527-1532.
- Smith IE, Dowsett M, Ebbs SR, Dixon JM, Skene A, et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the immediate preoperative anastrozole, tamoxifen, or combined with tamoxifen (impact) multicenter double-blind randomized trial. J clin oncol. 2005;23:5108-5116.
- Dixon JM, Renshaw L, Macaskill EJ, Young O, Murray J, et al. Increase in response rate by prolonged treatment with neoadjuvant letrozole. Breast cancer res treat. 2009;113:145-151.
- Lightowlers SV, Boersma LJ, Fourquet A, Kirova YM, Offersen BV, et al. Preoperative breast radiation therapy: Indications and perspectives. Eur J Cancer. 2017;82:184-192.
- Bosma SC, Leij F, Vreeswijk S, de Maaker M, Wesseling J, et al. Five-year results of the preoperative accelerated partial breast irradiation (PAPBI) trial. Int J Radiat Oncol Biol Phys. 2020;106:958-967.
- Cleator S, Leff D, Yarnold J. Emergency guidelines for pre-operative breast radiotherapy during the COVID-19 pandemic.
- Brunt AM, Haviland JS, Wheatley DA, Sydenham MA, Alhasso A, et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet. 2020;395:1613-1626.
- Tang N, Bai H, Chen X, Gong J, Li D, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J thromb haemost. 2020;18:1094-1099.
- Al-Quteimat OM, Amer AM. The impact of the COVID-19 pandemic on cancer patients. Am j clin oncol. 2020.
- Earl HM, Hiller L, Vallier AL, Loi S, McAdam K, et al. 6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet. 2019;393:2599-2612.
- Coles CE, Aristei C, Bliss J, Boersma L, Brunt AM, et al. International guidelines on radiation therapy for breast cancer during the COVID-19 pandemic. Clin Oncol (R Coll Radiol (G B). 2020;32:279.
- Brewster AM, Chavez-MacGregor M, Brown P. Epidemiology, biology, and treatment of triple-negative breast cancer in women of African ancestry. Lancet Oncol. 2014;15:e625-634.
- Brunt AM, Haviland J, Sydenham M, Algurafi H, Alhasso A, et al. FAST phase III RCT of radiotherapy hypofractionation for treatment of early breast cancer: 10-year results (CRUKE/04/015). Int J Radiat Oncol Biol Phys. 2018;102:1603-1604.
- Bloomfield DJ. On behalf of the core group facilitated by the royal college of radiologists. Development of postoperative radiotherapy for breast cancer: UK consensus statements-a model of patient, clinical and commissioner engagement. Clin Oncol. 2017;29:639-641.



