Research Article - Onkologia i Radioterapia ( 2023) Volume 17, Issue 9
The diagnostic accuracy of CT-guided biopsy in the diagnosis of lung cancer
Zahir Salih Khoshnaw1, Hemin Khalid Saber2 and Ayad Sameen Faraj3*2Internal Medicine, Pulmonology Specialist, Medical Department, Hawler Medical University, Erbil, Kurdistan Region, Iraq
3Internist, Respiratory Board Student, Rizgary Teaching Hospital, Erbil, Kurdistan Region, Iraq
Ayad Sameen Faraj, Internist, Respiratory Board Student, Rizgary Teaching Hospital, Erbil, Kurdistan Region, Iraq, Email: ayadsameen@gmail.com
Received: 05-Jul-2023, Manuscript No. OAR-23-104847; Accepted: 28-Jul-2023, Pre QC No. OAR-23-104847 (PQ); Editor assigned: 18-Jul-2023, Pre QC No. OAR-23-104847 (PQ); Reviewed: 29-Jul-2023, QC No. OAR-23-104847 (Q); Revised: 24-Aug-2023, Manuscript No. OAR-23-104847 (R); Published: 03-Sep-2023
Abstract
Background: Lung cancer is the second most common cancer worldwide, with higher rates of incidence among men and seniors. CT scan-guided biopsy has been known to be a good and less invasive test for diagnosing lung cancer. The present study was carried out to examine the diagnostic accuracy of CT scan-guided biopsy in diagnosing lung cancer. Patients and Methods: One hundred patients suspected of lung cancer were studied in a prospective observational study conducted in Rizgary Teaching Hospital and outpatient respiratory private clinic-Hawler, Kurdistan/Iraq. A convenient sampling method was employed to select the patients. Required data were collected from the patient’s medical profiles and CT scan images. Results: Most of the patients were aged 70 to 79 years (40%), 50 to 59 years (23%), and 60 to 69 years (20%). Three-fourths of the patients (75%) were males. The location of lung cancer in most of the patients (63%) was peripheral, and the lesion size in 51% of the cases was more than 5 cm and 3 to 5 cm in 38% of them. Lung nodules had irregular margins in most of the cases (92%). The metastasis locations included the brain in 5 patients, the liver in 3 patients, the adrenal in 2 patients, bone in 2 patients, the liver and adrenal in 2 patients, and the brain and adrenal in 1 patient. Ct scan-guided was associated with no complications. The histopathology results were found to be statistically associated with the shape of the nodule margins (p-value=0.002). Conclusion: CT scan-guided lung biopsy can be used as a safe and reliable method for diagnosing lung cancer. This method has a very low rate of complication and high diagnostic accuracy.
Keywords
lung cancer, CT-guided biopsy, diagnostic accuracy, lung mass
Introduction
Lung cancer is the second most common cancer worldwide, and it is the first and second most common cancer among men and women respectively. In 2020, there were over 2.2 million newly diagnosed cases of lung cancer reported globally [1]. Findings from a recent investigation by Hussain and colleagues (2021) reveal a noteworthy increase in the prevalence rate of lung cancer in Iraq, rising from 4.08 to 5.60 per 100,000 individuals [2]. The male-to-female ratio was observed to be 3:1, and a greater frequency of lung cancer was found among elderly individuals. Lung cancer is classified as Non-Small Cell Lung Cancer (NSCLC) and Small Cell Lung Cancer (SCLC), which are distinguished by the histological characteristics of the originating cells [3]. Lung Tumours that are generally larger than three centimetres (1.2 inches) are called pulmonary masses while, if it is three centimetres or less in diameter, it is commonly called pulmonary nodule [4, 5].
The precise classification of lung nodules is pivotal in determining the optimal course of treatment and management [6]. The size of pulmonary nodules is frequently utilized as a key determinant in the assessment of malignant potential, and as such, is commonly employed as a categorization method. The magnitude of a lung nodule is typically established through radiographic imaging techniques, such as Computed Tomography (CT) scans, and is commonly expressed in millimetres (mm) [5].
Nodules that measure below 6 mm in diameter are considered small and exhibit a lower propensity toward malignancy. Conversely, nodules ranging in size from 6 mm to 10 mm are classified as intermediate; these are more likely to be malignant and may require further evaluation, such as repeat imaging or biopsy [6]. Nodules exceeding 10 mm in diameter are categorized as large and have the greatest, probability of malignancy than other nodules and may necessitate further evaluation or intervention, such as biopsy, surgical resection, or radiation therapy [7].
Nevertheless, the probability of malignancy in a lung nodule cannot be determined by size alone but additional factors, such as morphology, texture, and growth kinetics, must also be taken into account [8].
The presence of pulmonary masses (>3 cm in diameter) is a significant indication of lung cancer [9].
A comprehensive and multidisciplinary approach including oncologists, pulmonologists, and radiologists is required for the diagnosis of lung cancer [10]. In recent years, the rate of lung cancer diagnosis has shown a consistent increase, primarily due to heightened public health awareness and advancements in various imaging modalities [11].
Besides history, clinical examination, laboratory tests, and bronchoscopy, CT-guided biopsy has been demonstrated to be a secure and efficient technique for diagnosing lung cancer, with diagnostic accuracy estimates ranging from 70% to 95% [12].
The precision of CT-guided biopsy in identifying pulmonary masses is contingent on a range of variables, such as the size and positioning of the mass, the expertise of the radiologist administering the procedure, and the quality of the imaging equipment employed. Nonetheless, research indicates that CTguided biopsy can be a dependable and efficient ap proach for diagnosing lung masses, with reported success rates reaching as high as 95% in certain instances [13].
Several studies have reported variable diagnostic accuracies for CT-guided biopsy in the diagnosis of lung cancer. Some investigations have cited a combined sensitivity and specificity of 94% and 100%, respectively, for CT-guided biopsy in detecting lung cancer. Conversely, other studies have recorded a combined sensitivity and specificity of 96% and 99%, respectively, for CTguided biopsy in the diagnosis of lung cancer [14].
In addition to its high diagnostic accuracy, CT-guided biopsy has several advantages over other diagnostic techniques for lung cancer, including its minimally invasive nature, shorter procedure time, and lower risk of complications. The CT-guided biopsy is also more cost-effective compared to other diagnostic techniques, such as surgical biopsy [15]. In this regard, the present study was conducted to assess the diagnostic accuracy of CT-guided biopsy in the diagnosis of lung cancer.
Patients & Methods
Study design and setting
The present study was a prospective observational study which was carried out at Rzgary Teaching hospital and outpatient respiratory private clinic-Hawler, Kurdistan/Iraq from 1st November 2022 to 1st June 2023.
Study sample and sampling methods
The study sample consisted of 100 patients who were suspected of lung cancer by history, examination, and/or chest CT scan. The patients were selected by a convenient sampling method. For this purpose, patients suspected of lung cancer by CT scan findings and those who signed informed consent forms were included in the study, while patients with bleeding tendencies, those with severe respiratory, renal, liver, and heart failures, and those with pneumothorax were excluded from the study.
Data collection
Required data including the patients' sociodemographic, including age and sex, cancer location, lesion size, shape, and metastasis were collected. Biopsy was taken with cooperation between a pulmonologist and radiologist and samples were examined by two expert pathologists, then results of biopsy were collected and studied.
Data analysis
The collected data were analysed through Statistical Package for the Social Sciences (SPSS version 25.0) using descriptive statistics and the Chi-square test.
Ethical consideration
The study protocol was approved by the Kurdistan Board for Medical Specialties. Moreover, informed consent was obtained from the patients who were provided with sufficient information about the study’s aim and duration.
Results
In this study 100 patients with lung cancer were studied which are diagnosed mainly by CT-guided biopsy. According to the results, most of the patients (75%) were males, and the rest (25%) were females (Table 1).
Tab. 1. The patients' sex, cancer location and site, and lesion size
| Frequency (N) | Percentage (%) | |
|---|---|---|
|
Sex |
||
| Male | 75 | 75 |
| Female | 25 | 25 |
| Total | 100 | 100 |
|
Location |
||
| Central | 37 | 37 |
| Peripheral | 63 | 63 |
| Total | 100 | 100 |
|
Site |
||
| Lingula | 2 | 2 |
| LLL | 17 | 17 |
| LUL | 28 | 28 |
| RLL | 19 | 19 |
| RML | 5 | 5 |
| RUL | 28 | 28 |
| RUL, LLL, and RML | 1 | 1 |
| Total | 100 | 100 |
|
Lesion Size |
||
| 1 cm-3 cm | 11 | 11 |
| 3 cm-5 cm | 38 | 38 |
| >5 cm | 51 | 51 |
| Total | 100 | 100 |
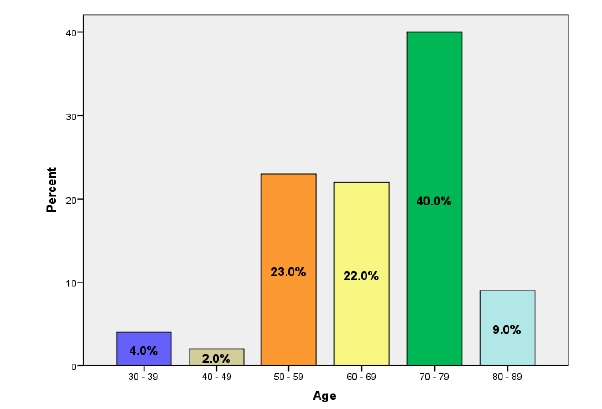
According to the results, most of the patients (40%) were aged 70 years to 79 years, followed by those who aged 50 years to 59 years (23%), 60 years to 69 years (22%), 80 years to 89 years (9%), 30 years to 39 years (4%), and 40 years to 49 years (2%) (Figure 1).
Figure 1: The patients' age
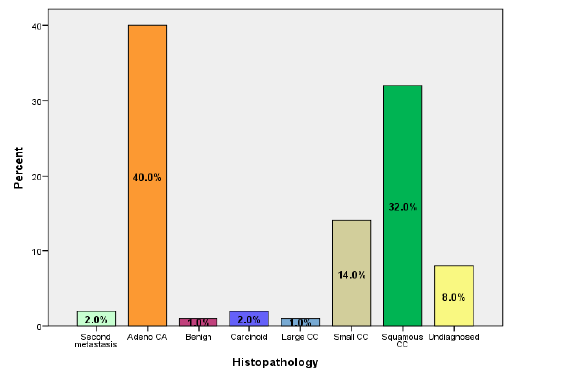
According to the results of the histopathology, adenocarcinoma was seen in 40% of the patients, squamous cell carcinoma in 32%, small-cell carcinoma in 14%, second metastasis in 2%, carcinoid in 2%, large-cell carcinoma in 1%, and benign in 1%. Lung cancer was not diagnosed in 8% (Figure 2).
Figure 2: The histopathology results
Regarding the location of lung cancer, it was seen that it was peripheral in most cases (63%) and central in over one-third of them (37%). The results indicated that RUL and LUL each in 28% of the patients were the most frequently involved sites, followed by RLL in 19%, LLL in 17%, RML in 5%, and lingua in 2%. The lesion size was over 5 cm in 51% of the patients, 3 cm to 5 cm in 38%, and 1 cm to 3 cm in 11% (Table 1).
The shape of lung nodules had irregular margins in most cases (92%) and regular margins in 8% of them. The associated findings were effusion in 10% of the patients, collapse in 5%, and effusion and collapse in 5%. Lung cancer metastasized to the brain in 5% of the patients, to the liver in 3%, to the adrenal in 2%, to bone in 2%, to liver and adrenal in 2%, and to brain and adrenal in 1%. A complication of biopsy was seen in none of the patients (Table 2).
Tab. 2. Histopathology results of the lung cancer
| Frequency (N) | Percentage (%) | |
|---|---|---|
| Shape | ||
| Irregular Margin | 92 | 92 |
| Regular Margin | 8 | 8 |
| Total | 100 | 100 |
| Associated Finding | ||
| Collapse | 5 | 5 |
| Effusion | 10 | 10 |
| Effusion and Collapse | 5 | 5 |
| None | 80 | 80 |
| Total | 100 | 100 |
| Metastasis | ||
| Adrenal | 2 | 2 |
| Bone | 2 | 2 |
| Brain | 5 | 5 |
| Brain and adrenal | 1 | 1 |
| Liver | 3 | 3 |
| Liver and adrenal | 2 | 2 |
| None | 85 | 85 |
| Total | 100 | 100 |
| Complication of biopsy | ||
| None | 100 | 100 |
As the results of the present study revealed, the obtained histopathology results did not have any significant associations with the patients' sex or age (p-value>0.05) (Table 3).
Tab. 3. Association between the patients' sex and age and histopathology results.
| Variables | Histopathology | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Second metastasis | Adeno CA | Benign | Carcinoid | Large CC | Small CC | Squamous CC | Undiagnosed | Total | P-value | |
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | ||
| Sex | ||||||||||
| Male | 2(100.0) | 28(70.0) | 1(100.0) | 2(100.0) | 0(0.0) | 9(64.3) | 28(87.5) | 5(62.5) | 75(75.0) | 0.21 |
| Female | 0(0.0) | 12(30.0) | 0(0.0) | 0(0.0) | 1(100.0) | 5(35.7) | 4(12.5) | 3(37.5) | 25(25.0) | |
| 2(100.0) | 40(100.0) | 1(100.0) | 2(100.0) | 1(100.0) | 14(100.0) | 32(100.0) | 8(100.0) | 100(100.0) | ||
| Age | ||||||||||
| 30 - 39 | 0(0.0) | 2(5.0) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 1(3.1) | 1(12.5) | 4(4.0) | 0.56 |
| 40 - 49 | 0(0.0) | 1(2.5) | 0(0.0) | 1(50.0) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 2(2.0) | |
| 50 - 59 | 0(0.0) | 12(30.0) | 0(0.0) | 0(0.0) | 0(0.0) | 3(21.4) | 6(18.8) | 2(25.0) | 23(23.0) | |
| 60 - 69 | 0(0.0) | 9(22.5) | 1(100.0) | 0(0.0) | 1(100.0) | 5(35.7) | 5(15.6) | 1(12.5) | 22(22.0) | |
| 70 - 79 | 2(100.0) | 12(30.0) | 0(0.0) | 1(50.0) | 0(0.0) | 6(42.9) | 15(46.9) | 4(50.0) | 40(40.0) | |
| 80 - 89 | 0(0.0) | 4(10.0) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 5(15.6) | 0(0.0) | 9(9.0) | |
| Total | 2(100.0) | 40(100.0) | 1(100.0) | 2(100.0) | 1(100.0) | 14(100.0) | 32(100.0) | 8(100.0) | 100(100.0) | |
According to the results, the histopathology results were not significantly associated with the lesion site, location, or size (p-value>0.05) (Table 4).
Tab. 4. Association between histopathology results and lesions site, location, and size
| Variables | Histopathology | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Second metastasis | Adeno CA | Benign | Carcinoid | Large CC | Small CC | Squamous CC | Undiagnosed | Total | P-value | |
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | ||
| Site | ||||||||||
| Lingula | 0(0.0) | 2(5.0) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 2(2.0) | 0.46 |
| LLL | 0(0.0) | 5(12.5) | 1(100.0) | 0(0.0) | 0(0.0) | 4(28.6) | 3(9.4) | 4(50.0) | 17(17.0) | |
| LUL | 0(0.0) | 10(25.0) | 0(0.0) | 0(0.0) | 1(100.0) | 5(35.7) | 10(31.3) | 2(25.0) | 28(28.0) | |
| RLL | 0(0.0) | 10(25.0) | 0(0.0) | 2(100.0) | 0(0.0) | 1(7.1) | 6(18.8) | 0(0.0) | 19(19.0) | |
| RML | 0(0.0) | 3(7.5) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 2(6.3) | 0(0.0) | 5(5.0) | |
| RUL | 2(100.0) | 10(25.0) | 0(0.0) | 0(0.0) | 0(0.0) | 4(28.6) | 10(31.3) | 2(25.0) | 28(28.0) | |
| RUL, LLL and RML | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 1(3.1) | 0(0.0) | 1(1.0) | |
| Total | 2(100.0) | 40(100.0) | 1(100.0) | 2(100.0) | 1(100.0) | 14(100.0) | 32(100.0) | 8(100.0) | 100(100.0) | |
| Location | ||||||||||
| Central | 0(0.0) | 14(35.0) | 0(0.0) | 2(100.0) | 1(100.0) | 9(64.3) | 8(25.0) | 3(37.5) | 37(37.0) | 0.041 |
| Peripheral | 2(100.0) | 26(65.0) | 1(100.0) | 0(0.0) | 0(0.0) | 5(35.7) | 24(75.0) | 5(62.5) | 63(63.0) | |
| Total | 2(100.0) | 40(100.0) | 1(100.0) | 2(100.0) | 1(100.0) | 14(100.0) | 32(100.0) | 8(100.0) | 100(100.0) | |
| Lesion Size | ||||||||||
| 1 - 3 cm | 0(0.0) | 6(15.0) | 0(0.0) | 0(0.0) | 0(0.0) | 1(7.1) | 1(3.1) | 3(37.5) | 11(11.0) | 0.24 |
| 3 - 5 cm | 2(100.0) | 16(40.0) | 1(100.0) | 1(50.0) | 0(0.0) | 6(42.9) | 10(31.3) | 2(25.0) | 38(38.0) | |
| > 5 cm | 0(0.0) | 18(45.0) | 0(0.0) | 1(50.0) | 1(100.0) | 7(50.0) | 21(65.6) | 3(37.5) | 51(51.0) | |
| Total | 2(100.0) | 40(100.0) | 1(100.0) | 2(100.0) | 1(100.0) | 14(100.0) | 32(100.0) | 8(100.0) | 100(100.0) | |
The results of the study indicated that the results of histopathology had significant associations with the lung cancer location (p-value=0.041) and the shape of lung nodules (p-value=0.002). However, they had no significant relationships with metastasis and associated findings (p-value>0.05) (Table 5).
Tab. 5. Association between histopathology results and other studied results
| Variables | Histopathology | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Second metastasis | Adeno CA | Benign | Carcinoid | Large CC | Small CC | Squamous CC | Undiagnosed | Total | P-value | |
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | ||
| Associated Finding | ||||||||||
| Collapse | 0(0.0) | 1(2.5) | 0(0.0) | 1(50.0) | 0(0.0) | 1(7.1) | 2(6.3) | 0(0.0) | 5(5.0) | 0.59 |
| Effusion | 0(0.0) | 5(12.5) | 0(0.0) | 0(0.0) | 0(0.0) | 3(21.4) | 2(6.3) | 1(12.5) | 11(11.0) | |
| Effusion and Collapse | 0(0.0) | 1(2.5) | 0(0.0) | 0(0.0) | 0(0.0) | 1(7.1) | 1(3.1) | 1(12.5) | 4(4.0) | |
| None | 2(100.0) | 33(82.5) | 1(100.0) | 1(50.0) | 1(100.0) | 9(64.3) | 27(84.4) | 6(75.0) | 80(80.0) | |
| Total | 2(100.0) | 40(100.0) | 1(100.0) | 2(100.0) | 1(100.0) | 14(100.0) | 32(100.0) | 8(100.0) | 100(100.0) | |
| Metastasis | ||||||||||
| Adrenal | 0(0.0) | 1(2.5) | 0(0.0) | 0(0.0) | 0(0.0) | 1(7.1) | 0(0.0) | 0(0.0) | 2(2.0) | 0.62 |
| Bone | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 2(6.3) | 0(0.0) | 2(2.0) | |
| Brain | 0(0.0) | 3(7.5) | 0(0.0) | 0(0.0) | 0(0.0) | 1(7.1) | 1(3.1) | 0(0.0) | 5(5.0) | |
| Brain and adrenal | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 1(3.1) | 0(0.0) | 1(1.0) | |
| Liver | 0(0.0) | 2(5.0) | 0(0.0) | 0(0.0) | 0(0.0) | 1(7.1) | 0(0.0) | 0(0.0) | 3(3.0) | |
| Liver and adrenal | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 1(7.1) | 0(0.0) | 1(12.5) | 2(2.0) | |
| None | 2(100.0) | 34(85.0) | 1(100.0) | 2(100.0) | 1(100.0) | 10(71.4) | 28(87.5) | 7(87.5) | 85(85.0) | |
| Total | 2(100.0) | 40(100.0) | 1(100.0) | 2(100.0) | 1(100.0) | 14(100.0) | 32(100.0) | 8(100.0) | 100(100.0) | |
| Shape | ||||||||||
| Irregular Margin | 2(100.0) | 39(97.5) | 0(0.0) | 1(50.0) | 1(100.0) | 14(100.0) | 30(93.8) | 5(62.5) | 92(92.0) | 0.002 |
| Regular Margin | 0(0.0) | 1(2.5) | 1(100.0) | 1(50.0) | 0(0.0) | 0(0.0) | 2(6.3) | 3(37.5) | 8(8.0) | |
| Total | 2(100.0) | 40(100.0) | 1(100.0) | 2(100.0) | 1(100.0) | 14(100.0) | 32(100.0) | 8(100.0) | 100(100.0) | |
Discussion
The results of our study indicated that adenocarcinoma and squamous cell carcinoma were observed in more than twothirds and nearly two-thirds of the patients, respectively. Unlike this finding, Barclay et al (2019) reported increased incidence of subsequent lung, laryngeal, head and neck, and oesophageal squamous cell carcinoma for at least a decade from the first diagnosis among lung cancer survivors [16]. Moreover, Miller et al (2011) reported that adenocarcinoma of the lung usually evolves from the mucosal glands and represents about 40% of all lung cancers [17].
In the present study, three-fourths of the patients were a male, which shows a higher percentage of lung cancer in men than in women. In line with this finding of the present study, Hellyer et al (2019) reported that the chance that a 1 out of 16 men develop lung cancer in his lifetime, while the rate is around 1 in 17 women [18]. These figures are true for both smokers and non-smokers. They also stated that the risk was much higher among smokers [19]. In another similar study, Thandra et al (2021) reported that the age-standardized cumulative lifetime risk of developing lung cancer was 1.77% among women and 3.8% among men [20]. They also pointed out that developing nation in which cigarette smoking is most prevalent have the highest incidence of lung cancer, with over a 20-fold variation in incidence between regions.
According to the results of the current study, in nearly twothirds of the cases, lung cancer was peripheral which was higher compared with the central ones. Similar to our study, Moon et al (2016) reported that the lung cancer type in the majority of the patients was peripheral rather than central [21]. The results of another study by Ock et al (2014) demonstrated that the preferential occurrence of the peripheral type of lung cancer was associated with the major histopathologic type of lung cancer, adenocarcinoma and unclassifiable non-small-cell lung cancer, and small-cell carcinoma [22].
More than 90% of the patient’s lesion size was larger than 3 cm, considered lung masses. In a similar study, Gould et al (2013) reported that lesions larger than 3 cm in diameter are called lung masses and are usually considered malignant [8]. Their study suggests that the detection of lung masses on imaging studies is a significant finding that necessitates further evaluation to determine their nature as benign or malignant. As such, it is crucial to obtain an accurate diagnosis and provide prompt treatment to enhance outcomes for individuals with lung masses or other imaging findings that arouse suspicion [4].
In the present study, lung nodules had irregular margins in more than 90% of cases. In line with this finding, Gould et al (2013) reported that malignant lung cancer is five times more likely to be diagnosed for nodules that have ragged or spiculated margins, around twice as likely when pleural retraction is present, and 70% more likely when a vessel sign was present but only 10% more likely when margins were lobulated [8].
Based on the results of our study, effusion was observed in 10% of lung cancer patients. In a similar study by Hardavella et al (2020), over one-third of the cases with malignant pleural effusion were secondary to lung cancer [23]. They also observed pleural effusions in 15% of lung cancer patients at the time of diagnosis, which might rise to 50% over time. Our study further revealed that the most common areas for lung cancer to spread are the brain, liver, bone, and adrenal glands. This finding is in line with those reported by Popper (2016) who pointed out that lung cancer metastasizes to the adrenal glands, the liver, bones, the brain, and nearby lymph nodes or a single distant lymph node [24]. Based on the results of our study, there was a significant association between the location of lung cancer and the shape of lung nodules with the probability of malignancy. In line with the data achieved from the present study, Heuvelmans et al (2017) reported that compared to benign baseline nodules, malignant nodules were larger and more often sub-solid, had more often a non-smooth margin, and were more often located in the upper lobes of the lung [25]. In another similar study conducted by Heidinger et al (2017), a malignancy prevalence rate of 2.3% to 6% was reported for nodules with a diameter of 5 mm to 9 mm, which is also in line with the present study's findings [26].
Conclusion
CT-guided biopsy of a lung lesion is safe and reliable, with a relatively low risk of complications and a high level of accuracy in diagnosing lung cancer. Lung cancer is more common in men than women and more peripheral than central. Irregular lung nodules have a higher risk of malignancy
References
- Miller KD, Nogueira L, Devasia T, Mariotto AB, Yabroff KR, et al. Cancer treatment and survivorship statistics, 2022. CA: cancer j clin. 2022; 72:409-436.
- Hussain AM, Lafta RK. Cancer trends in Iraq 2000–2016. Oman med j. 2021;36:219.
- Raso MG, Bota-Rabassedas N, Wistuba II. Pathology and Classification of SCLC. Cancers. 2021;13:820.
- Khan T, Usman Y, Abdo T, Chaudry F, Keddissi JI, Youness HA. Diagnosis and management of peripheral lung nodule. Ann Transl Med. 2019;15.
- Larici AR, Farchione A, Franchi P, Ciliberto M, Cicchetti G, et al. Lung nodules: size still matters. Eur respir rev. 2017;26.
- Loverdos K, Fotiadis A, Kontogianni C, Iliopoulou M, Gaga M. Lung nodules: a comprehensive review on current approach and management. Ann thorac med. 2019;14:226.
- Mazzone PJ, Lam L. Evaluating the patient with a pulmonary nodule: a review. Jama. 2022;327:264-273.
- Gould MK, Donington J, Lynch WR, Mazzone PJ, Midthun DE, et al. Evaluation of individuals with pulmonary nodules: When is it lung cancer?: Diagnosis and management of lung cancer: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:93-120.
- Panunzio A, Sartori P. Lung cancer and radiological imaging. Curr radiopharm. 2020;13:238-242.
- Margerie-Mellon C, Bazelaire D C, De Kerviler E. Image-guided biopsy in primary lung cancer: Why, when and how. Diagn interv imaging. 2016;97:965-972. [Google Scholar]
[CrossRef]
- Callister ME, Baldwin DR, Akram AR, Barnard S, Cane P, et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules: accredited by NICE. Thorax. 2015;70:1-54.
- Wu D, Liu YY, Wang T, Huang YY, Xia P. Computed tomography-guided lung biopsy with rapid on-site evaluation for diagnosis of lung lesions: a meta-analysis. J Cardiothorac Surg. 2023;18:1-8.
- Najafi A, Al Ahmar M, Bonnet B, Delpla A, Kobe A, et al. The PEARL approach for CT-guided lung biopsy: assessment of complication rate. Radiology. 2022;302:473-480.
- Mitchell CL, Zhang AL, Bruno DS, Almeida FA. NSCLC in the Era of Targeted and Immunotherapy: What Every Pulmonologist Must Know. Diagnostics. 2023;13:1117. [Google Scholar]
[CrossRef]
- Nakamura K, Matsumoto K, Inoue C, Matsusue E, Fujii S. Computed Tomography-guided Lung Biopsy: A Review of Techniques for Reducing the Incidence of Complications. Interv Radiol. 2021;6:83-92.
- Barclay ME, Lyratzopoulos G, Walter FM, Jefferies S, Peake MD, et al. Incidence of second and higher order smoking-related primary cancers following lung cancer: a population-based cohort study. Thorax. 2019;74:466-472.
- Xu YJ, Shi ZL, Wang Z, Du L, Cheng SZ, et al. Gefitinib in the Treatment of a Pulmonary Sequestration Patient Complicated by Large Cell Lung Carcinoma. J Thorac Oncol. 2018;13:165-168.
- Hellyer JA, Patel MI. Sex disparities in lung cancer incidence: validation of a long-observed trend. Transl Lung Cancer Res. 2019;8:543.
- Tolwin Y, Gillis R, Peled N. Gender and lung cancer—SEER-based analysis. Ann Epidemiol. 2020;46:14-19.
- Souliotis K, Golna C, Golnas P, Markakis IA, Linardou H, et al. Lung Cancer Screening in Greece: A Modelling Study to Estimate the Impact on Lung Cancer Life Years. Cancers. 2022;14:5484.
- Moon Y, Lee KY, Sung SW, Park JK. Differing histopathology and prognosis in pulmonary adenocarcinoma at central and peripheral locations. J thorac dis. 2016;8:169.
- Ock SY, Jang TW, Han YJ, Yeo GE, Kim EJ, et al. Characteristics of peripheral versus central lung cancer since 2000. Kosin Med J. 2014;29:47-52.
- Hardavella G, Karampinis I. Primary lung cancer and pleural effusion—diagnostic and therapeutic approach. AME Med J. 2020;5.
- Popper HH. Progression and metastasis of lung cancer. Cancer Metastasis Rev. 2016;35:75-91.
- Heuvelmans MA, Walter JE, Peters RB, de Bock GH, Yousaf-Khan U, et al. Relationship between nodule count and lung cancer probability in baseline CT lung cancer screening: The NELSON study. Lung Cancer. 2017;113:45-50.
- Heidinger BH, Anderson KR, Nemec U, Costa DB, Gangadharan SP, et al. Lung adenocarcinoma manifesting as pure ground-glass nodules: correlating CT size, volume, density, and roundness with histopathologic invasion and size. J Thorac Oncol. 2017;12:1288-1298.