Research Article - Onkologia i Radioterapia ( 2020) Volume 14, Issue 3
The effect of continuous infusion of meropenem antibiotic on clinical signs and changes of procalcitonin in patients with acute sepsis related to cancer
Javad Farokhi1, Alireza Kamali1, Nader Zarrinfar2, Behnam Mahmodiyeh1*, Bijan Yazdi1 and Mohammad Jamalian12Department of Infectious Disease, Arak University of Medical Sciences, Arak, Iran
Behnam Mahmodiyeh, Department of Anesthesiology and Critical Care, Arak University of Medical Sciences, Arak, Iran, Email: behnam@gmail.com
Received: 24-Dec-2019 Accepted: 03-Jan-2020 Published: 09-Jan-2020
Abstract
Introduction: Infections in critically ill immunocompromised patients with cancer are related to different diagnostic and therapeutic challenges. Sepsis, severe sepsis, and septic shock are used to describe the body's systemic response to aggressive microorganisms, including bacteria and fungi. The aim of the present study was to evaluate the effect of continuous infusion of meropenem antibiotics on clinical signs and changes of procalcitonin in patients with acute sepsis.
Material and Methods: This study is a double-blind randomized clinical trial. The number of patients in each group was 30. In the infusion group, they received 1 gram of meropenem as a bolus and 100 mg (10 ml) of meropenem per hour of continuous intravenous infusion over a 72-hour period. Patients in the bolus group received one gram of meropenem as a bolus, followed by a bolus dose every 12 hours, as well as a placebo infusion of 10 ml/h for 72 hours. Patients' clinical symptoms including fever, hypertension, heart rate, respiratory rate, the severity of the disease, drug sensitivity, and level of consciousness at admission were recorded 24, 48 and 72 hours after drug administration. Data were analyzed by SPSS v19.
Results: There was a significant decrease in temperature in the infusion group 24, 48 and 72 hours after treatment (p<0.05). In the infusion group the heart rate was lower (p=0.001). The severity of the disease was better in the infusion group (p<0.05). The level of consciousness was better in the infusion group (p<0.05). 72 hours after treatment there was a statistically significant difference in white blood cell count (p<0.05). In the infusion group after 72 hours, the APACHE score was lower than in the bolus group (p<0.05).
Conclusion: The infusion group showed lower temperature, lower heart rate, higher consciousness, disease severity, and lower APACHE score.
Keywords
Treatment, APACHE score, sepsis, meropenem
Introduction
Sepsis remains a frequent complication in patients with cancer and associated with high mortality so, the immune pathophysiology of severe sepsis in cancer patients is mostly linked to immune deficiency imposed by anticancer treatments [1]. Systemic Inflammatory Response Syndrome is the systemic response of the body to fever, tachycardia, and leukocytosis, also called SIRS. Signs and symptoms of the disease include fever, increased heart rate, increased respiratory rate, and confusion. There are also other symptoms associated with certain infections, such as coughing with pneumonia or urinating with pain associated with kidney infection [1,2]. When SIRS occurs in a patient suspected of infection, it is called sepsis. If a patient has hypotension that is responsive to fluids or dysfunction in organs farther from the site of infection, the patient develops severe sepsis and septic shock can occur if lactic acidosis, or dysfunction of the vital organs of the body, or hypotension occurs with no response to fluids [1]. Sepsis or diffuse body response to infection, lung infections and AIDS is the third leading cause of death due to infection and as we look at increasing the trend, this syndrome is the most common cause of death in intensive care units. In the late 1980s, sepsis was the 13th leading cause of death in the United States, costing nearly 10 billion $ per year for medical treatment [3,4]. Estimates show that there are around 400,000 case of sepsis and about 200,000 septic shocks in the country a year, with about one 100,000 deaths reported [5]. Factors influencing the Increase of sepsis including an overdose of immune system suppressants, increase use of intravenous invasive devices, the average age of the population, and the recent increase in infections caused by resistant microorganisms. Despite the recent advances in pathogenesis and the presentation of the pathophysiology of sepsis and the introduction of highly potent antibacterial and antifungal drugs, there is still little success in definitively reducing the morbidity and mortality of this disorder. It should be noted that the reported mortality of patients with gram-negative sepsis is in the range of 20% to 80% [5]. The incidence of sepsis has been rising over the last 15 years. The key to treating sepsis is to identify early and start symptom-based treatment before complications such as hypotension occur. Systemic Inflammatory Response Syndrome (SIRS) in the early 1990s has been defined by intensive care professionals as having at least two of the following criteria (diagnosis criteria):
Fever or hypothermia, >20 min Tachypnea, >20 min Tachycardia, >12000 Leukocytosis or <4000 leukopenia or >10% bandemia [6].
Extended-spectrum antibiotic meropenem is a beta-lactam and carbapenem group that has remarkable resistance to hydrolysis by penicillinase and cephalosporins (excluding Metallo beta-lactamase) produced by Gram-positive and Gram-negative microorganisms. Meropenem is used to treat infections caused by Streptococcus viridans, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Bacteroides fragilis, Haemophilus influenzae and Neisseria meningitidis, this include intra-abdominal infections, meningitis, respiratory tract infections, septicemia, skin and soft tissue infections, and urinary tract infections. Meropenem inhibits bacterial cell wall synthesis. It penetrates the cell wall of most Gram-positive and Gram-negative bacteria and converts it to Penicillin-Binding Proteins (PBPs). As a result of inhibition of trans-peptidases, transpeptidation is impaired (cross-linking in the production of bacterial cell wall peptidoglycans). Finally, the autolytic enzymes, called murein hydrolases, (murein also called peptidoglycans), are activated in bacteria exposed to penicillin and cause the destruction of peptidoglycans. The result of this process is the destruction of the bacterial wall and the destruction of the bacterial cell [7]. Beta-lactam antibiotics are often administered in bolus doses [8]. However, pharmacodynamic data showed that continuous infusion of the drug may be more effective than bolus administration. The killing of bacteria by beta-lactam antibiotics depends on the time the bacterium is exposed to the antibiotic before the antibiotic level reaches its minimum level (T>MIC) [9].
Intravenous administration of beta-lactam antibiotics over and above the Minimum Inhibitory Concentration (MIC), and especially when administered intermittently, increases the concentration of antibiotics in the interstitial fluid, which is very common in the ICU [10]. Although in animal studies and in vitro studies, continuous injections were superior to intermittent use, 2 Meta-analyzes in human trials to date have shown no difference in clinical treatment or survival [11,12]. These human studies were primarily performed in critically ill patients and on the other hand, in 13 of the 14 infusion studies evaluated, uneven doses were compared in the two groups of Intermittent and continuous infusion therapy [13]. Also in this disease, the lack of a specific infection marker has led to problems in distinguishing SIRS from infectious and non-infectious agents, which in turn may lead to underuse or, in some cases, overuse of antimicrobial agents. Therefore, differential tests of noninfectious sepsis inflammation are very useful. For this reason, many studies have been done in the past decade to access markers that can be used to diagnose early sepsis [14]. The results of studies of PCT levels in patients with sepsis indicate that serum levels of procalcitonin are elevated in these patients. Therefore, its reduction indicates an improvement in patients with sepsis [15]. Procalcitonin as pro-hormone calcitonin is a 116 amino acid polypeptide that is secreted by C cells from the thyroid gland in response to hypercalcemia and is activated intercellularly with proteolytic enzymes. Procalcitonin during sepsis is produced by macrophages and monocytes of various organs and released into the bloodstream [15,16]. The blood concentration of pro-calcitonin in healthy individuals is lower than detectable and increases in systemic inflammation, especially bacterial infections [17] and is strongly associated with systemic bacterial infection [18].
The aim of this study was to compare the efficacy of two continuous infusions of meropenem and intravenous injection of meropenem with the use of marker of procalcitonin in the treatment of patients with sepsis.
Materials and Methods
The study was designed as a double-blind randomized clinical trial and the study population was selected from patients admitted to the intensive care unit of Valiasr Hospital in Arak with a diagnosis of evidence of infection and at least two cases of SIRS (Sepsis) and APACHE II score>15. In patients with inclusion criteria, different combinations of antibiotic treatments in addition to meropenem were prescribed in patients with different cases, the origin of infection, type of infection and clinical conditions of ICU patients. Inclusion criteria include the age range of 18 to 75 years, APACHE II SCORE>15, ICU patients diagnosed with evidence of infection and at least two cases of SIRS (sepsis) and consent to participate in the study. Exclusion criteria include an unwillingness to participate in the study, drug side effects such as renal failure, seizures, rash and skin rash, failure to respond within 72 hours.
Therefore, randomization based on the random numbers table prevented bias in the study. After diagnosis of sepsis, treatment with meropenem antibiotic was started and blood samples were taken from the patient and serum levels of procalcitonin (criteria for assessing patients with sepsis), blood saturation, creatinine, urea, platelet count, GFR (Glomerular Filteration Rate), and white blood cell count (WBC>11000 or <4000) were recorded before the start of treatment. Immediately after the initial diagnosis, patients in the infusion group (n=30) received 1 gram of meropenem (Dana Pharmaceutical Company, Iran) as a bolus at one time and 80 mg/hr of continuous infusion of meropenem for 72 hours. Also, a placebo vial (with the same volume) was injected as a bolus every 12 hours. Patients in the bolus group (n=30) received one gram of meropenem (Dana Pharmaceutical Company-Iran) as a bolus once every 12 hours and 10 ml/h placebo (normal saline) as a continuous infusion over a 72-hour period. It was the responsibility of the resident and the guidance and counseling professors. The clinical symptoms of the patients were as follows: fever, hypertension, heart rate, respiratory rate, drug sensitivity, and level of consciousness (Glasgow scale at baseline, 24, 48 and 72 hours after drug administration). The ventilator was measured and monitored by ventilator monitoring. After the end of drug treatment, all patients were re-sampled and the results were re-recorded and all patients were followed for two weeks after the end of treatment. At the end of the study, the final status of patients (72 hours) mortality, remission, and severity (based on Apache score) was recorded in the checklist. Other patient information including age, gender, number of ventilatordependent days, and length of stay in the ICU ward were recorded. Samples were divided into two groups of infusion and bolus in order to observe replication in terms of age, sex, and so on as the study was RCT and completely randomized. It should be noted that the patient was unaware of the treatment and blinded the analyzer and the patient (double-blind). Residents were also monitored for all sampling, clinical symptoms, and follow-up.
The collected data were analyzed by SPSS v.19 software package. Results were analyzed using mean, standard deviation, standard error, frequency percentage. An independent t-test or its parametric bread test was used for analysis.
Results
The study was designed as a double-blind randomized clinical trial. The study population was among patients admitted to the intensive care unit of Valiasr Hospital in Arak with a diagnosis of evidence of infection and at least two cases of SIRS (Sepsis) and APACHE II SCORE>15. The patients were randomly divided into two groups. The minimum age was 18 years and the maximum age was 75 years. The mean age of patients was 43.60 ± 17.95 years. None of the patients showed drug sensitivity. There was no significant difference between the two groups in age and sex (p=0.764) (Table 1).
| p-value | Bolus Mean ± SD | Infusion Mean ± SD | Group |
|---|---|---|---|
| 454/0 | 826/0 ± 62/38 | 682/0 ± 24/38 | Initiation |
| 004/0 | 643/0 ± 00/38 | 539 0 ± 54/37 | After 24 h |
| 0001/0 | 594/0 ± 81/37 | 513/0 ± 20/37 | After 48 h |
| 040/0 | 717/0 ± 53/37 | 37/1 ± 94/36 | After 72 h |
Table 1 : Comparison of the mean and standard deviation of temperature in infusion and bolus meropenem groups
According to the results, there was no statistically significant difference in the temperature between the two groups at first (p=0.454). 24, 48 and 72 hours after treatment in the infusion group the temperature was significantly decreased and this difference was statistically significant (p<0.05). There was no significant difference in systolic blood pressure, oxygen saturation and respiratory rate (p>0.05) between the two groups at different times (p>0.05). Heart rate was significantly different at 24, 48 and 72 hours after treatment (p=0.001). In the infusion group, heart rate was lower (Table 2).
| p-value | Bolus | Infusion | Group |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| 938/0 | 29/ 4 ± 50/10 | 26/4 ± 53/10 | Initiation |
| 003/0 | 79/3 ± 50/10 | 51/2 ± 06/12 | After 24 h |
| 001/0 | 71/3 ± 73/10 | 25/2 ± 76/12 | After 48 h |
| 006/0 | 42/3 ± 76/10 | 17/2 ± 23/13 | After 72 h |
Table 2 : Comparison of the mean and standard deviation of consciousness level between infusion and bolus meropenem groups
Results showed a statistically significant difference between the two groups regarding the level of consciousness during the treatment (p<0.05). The level of consciousness was better in the infusion group. During 72 hours, the consciousness level was better in the infusion group (Figure 1 and Table 3).
| p-value | Mean ± SD bolus | Mean ± SD infusion | Group | Lab parameter |
|---|---|---|---|---|
| 494/0 | 17/0 ± 258/0 | 17/0 ± 259/0 | Initiation | Procalcitonin |
| 732/0 | 034/0 ± 086/0 | 035/0 ± 087/0 | After 72h | |
| 172/0 | 326/0 ± 982/0 | 497/0 ± 970/0 | Initiation | Creatinine |
| 184/0 | 313/0 ± 948/0 | 45/0 ± 920/0 | After 72h | |
| 492/0 | 05/6 ± 46/17 | 86/8 ± 63/17 | Initiation | Urea |
| 342/0 | 50/7 ± 81/15 | 31/9 ± 06/18 | After 72h | |
| 262/0 | 13/35 ± 49/104 | 18/34 ± 56/105 | Initiation | GFR |
| 172/0 | 07/33 ± 06/103 | 19/50 ± 48/107 | After 72h | |
| 647/0 | 67/88 ± 80/217 | 53/88 ± 83/216 | Initiation | Platelets |
| 087/0 | 62/112 ± 43/301 | 14/78 ± 63/214 | After 72h | |
| 071/0 | 103 (69/44 ± 32/39) | 103 (09/35 ± 91/24) | Initiation | WBC |
| 002/0 | 103 (41/9 ± 72/15) | 103 (44/3 ± 24/11) | After 72h |
Table 3 : Comparison of mean and standard deviation of laboratory parameters in infusion and bolus meropenem groups
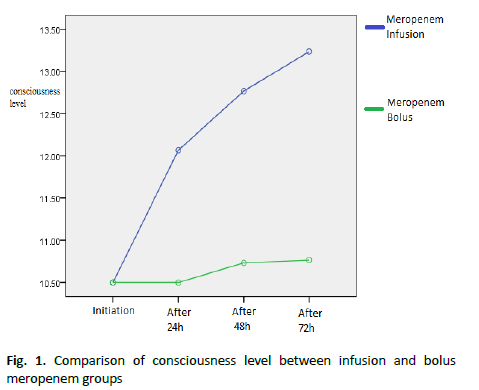
Figure 1. Comparison of consciousness level between infusion and bolus meropenem groups
According to the results 72 hours after treatment there was a statistically significant difference in white blood cell count (p<0.05). White cells were normal in the infusion group but in the bolus group, the white blood cells moved to normal but not in the normal range.
There was a significant difference between the two groups regarding the number of days of hospitalization and the number of days dependent on the ventilator (p<0.05). In the infusion group, the number of days hospitalized and the number of days dependent on the ventilator were lower than in the bolus group. In the bolus group, 2 cases of death were seen and there was a statistically significant difference in mortality (p=0.003) (Table 4).
| p-value | Mean ± SD Bolus | Mean ± SD Infusion | APACHE/ Group |
|---|---|---|---|
| 06/0 | 11/4 ± 23/20 | 72/4 ± 70/18 | Initiation |
| 005/0 | 37/4 ± 06/18 | 38/6 ± 96/13 | After 24 h |
| 014/0 | 08/5 ± 93/17 | 97/6 ± 93/13 | After 48 h |
| 020/0 | 86/5 ± 80/17 | 13/7 ± 76/13 | After 72 h |
Table 4 : Comparison of the mean and standard deviation of consciousness level between infusion and bolus meropenem groups
There was a statistically significant difference between the two groups in terms of APACHE results (p<0.05). In the infusion group after 72 hours, the APACHE score was lower than in the bolus group (Figure 2).
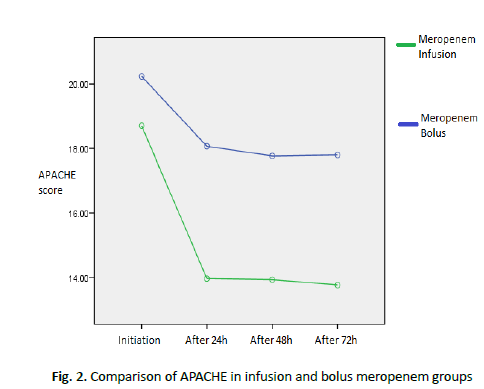
Figure 2. Comparison of APACHE in infusion and bolus meropenem groups
In the infusion group after 72 hours, the APACHE score was lower than in the bolus group. As can be seen, patients recovered faster in the infusion group.
Discussion
The study was designed as a double-blind randomized clinical trial and the study population was selected from patients admitted to the intensive care unit of Valiasr Hospital in Arak with a diagnosis of evidence of infection and at least two cases of SIRS (Sepsis) and APACHE II score>15. The subjects were randomly divided into two groups. There was no significant difference between the two groups in terms of age and gender (p>0.05). 24, 48 and 72 hours after treatment in the infusion group the temperature decreased and this difference was statistically significant (p<0.05). In the infusion group, the slope of the temperature decrease in the diagram was more than the bolus group. There was no significant difference in mean blood pressure between the two groups at a different time (p>0.05). In the infusion group, the heart rate was lower. There was no statistically significant difference between the two groups in terms of oxygen saturation and respiratory rate (p<0.05). The level of consciousness was better in the infusion group. During 72 hours the level of consciousness was better in the infusion group. Regarding the number of white blood cells in the infusion group, it was initially observed that after 72 hours the white blood cells in the infusion group normalized, but in the bolus group, the white blood cell count moved to normal but not in the normal range. In the infusion group, the number of days hospitalized and the number of days dependent on the ventilator were lower than in the bolus group. There were 2 deaths in the bolus group and there was a statistically significant difference in mortality (p= 0.003). In the infusion group after 72 hours, the Apache score was lower than in the bolus group. As can be seen, patients recovered faster in the infusion group. Here are some of the studies we have studied: A study titled "Continuous Infusion of Beta-Lactam Antibiotic in Patients with Severe Sepsis. Results showed that in patients receiving continuous administration of beta-lactam antibiotics (30 patients in each group), plasma antibiotic concentrations were higher than intermittent antibiotic patients and were associated with improved clinical treatment [13]. Their results were consistent with our study. In another study by Roberts in 2009, a study entitled "Drug therapy with meropenem in critically ill patients with sepsis without renal impairment: intermittent bolus versus continuous administration?" Monte Carlo drug simulation and subcutaneous tissue injection in Australia performed on 10 patients with sepsis. They demonstrated that the administration of meropenem with continuous infusion was superior to alternate bolus administration to maintain drug concentration in subcutaneous tissue and plasma was better. They reported that limited available data indicated that continuous infusion of β-lactam antibiotics resulted in clinical outcomes similar to bolus dose administration in hospitalized patients [12]. The results of our study showed a decrease in the severity of the disease and a decrease in the mortality in the infusion group. In our study, the effect of infusion was better in reducing the severity of the disease and improving the patients. The reason for the difference may be because Roberts et al.'s review study used a variety of beta-lactam antibiotics, while our study used meropenem antibiotics. A review study titled "Continuous Beta- Lactam Injection in Patients: Clinical Evidence" was conducted in 2012. The results of these trials indicate that continuous infusion of beta-lactam antibiotics may have variable efficacy in different patient groups. Patients with very high disease severity are patients who may benefit from this type of treatment [19]. Their results were in line with ours. A study entitled "Comparison of Continuous Beta-Lactam versus Intermittent Injection in Patients with Severe Sepsis: A Meta-Analysis of Randomized Trials" was conducted in 2016. The results showed a reduction in in-hospital mortality in patients receiving beta-lactam antibiotics with continuous infusion compared to patients with intermittent administration [11]. A study, "A Systematic Review Study on the Clinical Benefits of Continuous Administration of β-Lactam Antibiotics," was conducted by Kasiakou et al. (Athens-Greece) concluded that antibiotic administration with continuous intravenous infusion may be more effective in clinical efficacy compared to intermittent administration [20]. Their results are consistent with our study. A study titled "Long-Term Injections of Intermittent Bolus Antibiotics with β-Lactam for the Treatment of Acute Infections: Meta-Analysis" was conducted in 2014 in Singapore. A total of 2206 patients were studied in 22 studies (18 randomized clinical trials and 11 case-control studies). Compared with the intermittent bolus, long-term injections appear to be associated with a significant decrease in mortality and clinical improvement. The benefits of this method were statistically significant and significant in non-randomized studies, but not in RCTs [21]. The results of our study also indicate a better effect of infusion. Overall, it seems that the infusion of meropenem in critical patients leads to a more stable survival of vital signs and possibly a faster exit from severe sepsis. On the other hand, there were 2 cases of death in the bolus group, but no mortality was seen in the infusion group after 72 hours.
Conclusion
In the infusion group, the temperature decreased significantly, 48 and 72 hours after treatment. In the infusion group, the heart rate was lower. The level of consciousness was better in the infusion group. During 72 hours the level of consciousness was better in the infusion group. Regarding the number of white blood cells in the infusion group, the study first observed that after 72 hours the number of white blood cells in the infusion group was normal. But in the bolus group, the white blood cell count moved to normal but not within the normal range. In the infusion group, the number of days hospitalized and the number of days dependent on the ventilator was lower than in the bolus group. In the bolus group, 2 cases of death were seen. In the infusion group after 72 hours, the APACHE score was lower than in the bolus group. As can be seen, patients improved faster in the infusion group.
Ethical Considerations
This study is part of a thesis entitled Code of Ethics IR.ARAK.MU.REC.1396.2. The clinical trial code for this study is IRCT20141209020258N65.
Conflict of Interest
None
References
- Staudinger T, Pène F. Current insights into severe sepsis in cancer patients. Rev Bras Ter Intensiva. 2014;26:335-338.
- Stearns-Kurosawa DJ OM, Valentine C, Kurosawa S, Remick DG. The pathogenesis of sepsis. Annu Rev Pathol-Mech. 2011;6:19-48.
- Centers for Disease Control (CDC). Increase in national hospital discharge survey cases for septicemia. MMWR Morb Mortal Wkly Rep. 1990;39:31-34.
- Centers for Disease Control (CDC). National Center for Health Statistics. Mortality Patterns in United States. 2006;41:45.
- Parrillo JEPM, Natanson C, Suffredini AF, Danner RL, Cunnion RE, et al. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med. 1990;113:227-242.
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864-874.
- Kasten B. β-lactam antibiotics inhibit chloroplast division in moss (Physcomitrella patens) but not in tomato (Lycopersicon esculentum). J Plant Physiol. 1997;1-2:137-140.
- Dulhunty JM, Paterson D, Webb SA, Lipman J. Antimicrobial utilisation in 37 Australian and New Zealand intensive care units. Anaesth Intensive Care. 2011;39:231-237.
- Roberts JA, Kruger P, Paterson DL, Lipman J. Antibiotic resistance-what ’ s dosing got to do with it? Crit Care Med. 2008;36:2433-2440.
- Gonçalves-Pereira J, Póvoa P. Antibiotics in critically ill patients: a systematic review of the pharmacokinetics of β-lactams. Crit Care. 2011;15:R206.
- Kasiakou SK, Lawrence KR, Choulis N, Falagas ME. Continuous versus intermittent intravenous administration of antibacterials with time-dependent action: a systematic review of pharmacokinetic and pharmacodynamic parameters. Drugs. 2005;65:2499-2511.
- Roberts JA, Webb S, Paterson D, Ho KM, Lipman J. A systematic review on clinical benefits of continuous administration of beta-lactam antibiotics. Crit Care Med. 2009;37:2071-2078.
- Dulhunty JM, Roberts JA,Davis JS,Webb SA,Bellomo R, et al. Continuous infusion of beta-lactam antibiotics in severe sepsis: A multicenter double-blind, randomized controlled trial. Clin Infect Dis. 2013;56:236-244.
- Simon L,Gauvin F,Amre DK,Saint-Louis P,Lacroix J. Serum procalcitonin and c-reactive protein levels as markers of bacterial infection: A systematic review and meta-analysis. Clin Infect Dis. 2004;39:206-217.
- Chan YL, Tsay PK, Chang SS, Chiu TF, Chen JC. Procalcitonin as a marker of bacterial infection in the emergency department: An observational study. Crit Care. 2004;8:12-20.
- Muller B. Endocrine aspects of critical illness. Ann Endocrinol. 2007;68:290-298.
- Floriańczyk B. Structure and diagnostic value of procalcitonin. Ann Univ Mariae Curie Sklodowska Med. 2003;58:338-342.
- Christ-Crain M, Muller B. Procalcitonin in bacterial infections-hype, hope, more orless? Swiss Med Wkly. 2005;135:451-460.
- Abdul-Aziz MH, Dulhunty JM, Bellomo R, Lipman J, A Roberts J. Continuous beta-lactam infusion in critically ill patients: the clinical evidence. Ann Intensive Care. 2012;2:37.
- Kasiakou SK, MichalopoulosA, Soteriades ES, Falagas ME. Continuous versus intermittent intravenous administration of antibiotics: a meta-analysis of randomized controlled trials. Lancet Infect Dis. 2005;5:581-589.
- Teo J, Lee W, Kwa AL. Prolonged infusion versus intermittent boluses of beta-lactam antibiotics for treatment of acute infections: a meta-analysis. Int J Antimicrob Agents. 2014;43:403-411.



