Research Article - Onkologia i Radioterapia ( 2023) Volume 17, Issue 8
The human 8-oxoguanine DNA glycosylase and xeroderma pigmentosum group haplotypes variation in x- ray employees
Moayad Aziz Abdulqadir1*, Hussain Ghazil2, Mariam Alaa Toama3, Liwaa Hussein Mahdi4, Jamal A. Aljabbar Attawi5, Khulood Majid Alsaraf6 and Zahraa F. Hassan72Medical Lab techniques, College of Medical Technology, Al-Farahidi University, Iraq
3College of Pharmacy, National University of Science and Technology, Dhi Qar, Iraq
4Faculty of Medicine, University of Kufa, Iraq
5Al-Hadi University College, Baghdad, 10011, Iraq
6Al-Esraa University College, Baghdad, Iraq
7College of Dentistry, Al-Ayen University, Thi-Qar, Iraq
Moayad Aziz Abdulqadir, Department of Anesthesia Techniques, Al-Noor University College, Nineveh, Iraq, Email: moayad.aziz@alnoor.edu.iq
Received: 09-Jul-2023, Manuscript No. OAR-23-105528; Accepted: 10-Aug-2023, Pre QC No. OAR-23-105528 (PQ); Editor assigned: 11-Jul-2023, Pre QC No. OAR-23-105528 (PQ); Reviewed: 30-Jul-2023, QC No. OAR-23-105528 (Q); Revised: 07-Aug-2023, Manuscript No. OAR-23-105528 (R); Published: 14-Aug-2023, DOI: -
Abstract
DNA repair genes polymorphism including The human 8-oxoguanine DNA glycosylase (hOGG ) Ser326Cys and Xeroderma Pigmentosum Group (XPD) Arg399Gln genes in the x-ray employees were studied in present study. PCR-SSCP technique was used to detect haplotype polymorphisms. The results found that the haplotypes distribution of both genes were significant association with cases, XPD have high percentage of di haplotype than control group in significant association (p=0.0019).The hOGG haplotype distribution in study groups shows significant association of di haplotype with cases than control group (P=0.000), both genes haplotypes were distributed according work time in cases per day to more and less than 6 hours, XPD showed non-significant association XPD (p=0.9395), uni and di haplotype more frequent in cases exposure to x-ray less than 6 hours per day. The hOGG appeared in significant association with work time (p=0.000) that more frequent of di haplotype in less than 6 hours category, di haplotype more frequent in case work less than 6 hours per day. The di haplotypes more frequent in worker with less than 6 hours per day in non-significant association (p=0.4132). The exposure day to x-ray classified to less and more than 4 days per week, XPD non-significant association with exposure day (p=0.798) about 50% of worker have uni haplotype that work less than 4 days. The hOGG haplotypes also non-significant association with exposure day (p=0.798), Different work periods were reported and classified to less and more than 10 years, XPD showed non-significant association (p=0.582) with work period, uni haplotype was frequent in same percentage in both categories. The hOGG also non-significant correlations with work periods (p=0.1775), di haplotype more frequent in worker with less than 10 years, the current results concluded that there were vital role of x-ray in the hOGG Ser326Cys and (Arg399Gln) XPD genes.
Keywords
human 8-oxoguanine DNA glycosylase, xeroderma pigmentosum group, haplotypes, variation, x- ray employees
Introduction
The Ionizing Radiation (IR) disrupts chemical bonds by deposits energy on molecules that lead to breaks genomic DNA covalent bonds when it passes through cells. A wide variety of DNA damage were produced, like DNA single-strand breaks, base damage, double-strand breaks and DNA-protein crosslinks, furthermore the formation of oxidative stress promoted DNA damage by irradiation [1]. The double strand break number stimulated under normoxic conditions more than under hypoxic conditions [2, 3]. Different chemical modifications were formed during exposure of IR in hypoxic conditions like 5,6-dihydrothymine [4], α-deoxyadenosine (α-dA) [5], 5',8-cyclo-dA [6], and DNAprotein cross link [7, 8]. The Xeroderma Pigmentosum group D (XPD) gene, is found in chromosome 19q13.3. The XPD protein is important in the nucleotide excision repair pathway, with other functions, like the site of DNA lesions enzyme uncoiling the double helix and transcription[9], other studies have observed that variant alleles of XPD Lys751Gln (rs13181) polymorphism associated with DNA adduct levels increments [10,11,12], and DNA repair capacity reduction [13].
The Human oxoguanine glycosylase 1 or called (hOGG 1) is one of the DNA repair enzyme, that has a vital roles in the base excision repair pathway. Several studies reported a common polymorphism Ser326Cys (rs1052133) in hOGG 1 in different disease [14], the hOGG 1 enzyme is a major member of baseâ? excision repairing for damage of oxidative DNA [15]. It encoded by the hOGG 1 gene can directly remove 8â?hydroxyguanine (8â? OHâ?G), one of the major constituents in DNA damage [16,17, 18]
Methodology
Samples and study subjects
A case control study were implemented on the x-ray technicians that employees in Al-Sadder medical city, about 20 cases and 30 individuals as a control group, blood samples were collected according to ethical approval of ministry of health and environment in Iraq
DNA extraction and target genes amplifications
DNA isolated by favour gene kits with 40 µl of proteinase K (20 µg/µl). The hOGG Ser326Cys and XPD Arg399Gln genes amplified using primers that mention in previous study [18].
Haplotypes detected using SSCP technique according to the mixture consist of 40% of acrylamide-bis acrylamide 8 ml, glycerol 2.8 ml, TBE (5X) 8 ml and dH2 O 20.8 ml, ammonium-per sulfate 400 µl (0.1 gm/ml) and TEMD (40 µl) to gel casting, PCR products were mixed with loading dye (formamid, xylene cyanol, bromo- phenol blue with EDTA) in same ratio, then it incubate at 95ºC to 7 min, after that its chilled in ice for 2 min, next samples were loading in vertical casting and electrophoresis running at 100V, to 40 min, after electrophoresis finished gel was stained by ethidum bromide and visualized under UV light.
Data analysis
Haplotype was represented as percentage, significant detected by chi square at p-value less than 0.05.
Results
The results found that the age of cases was (36.1 years ± 10.5 years), the exposure time to x-ray was (4.09 years ± 2.99 years), and the work duration was (9.9 years ± 8.8 years). The present outputs of present study that deal with (Arg399Gln) in XPD and Ser326Cys in hOGG gene in the x-ray tecnitions that work in hospital, results of hOGG haplotypes found three haplotypes (uni, di and tri) in control and two haplotype (uni and di) in workers, haplotypes were visulized using PCR-SSCP technique (Figure 1A-C), the XPD have two haplotypes in both cases and control group (di and tri) (Figure 1D and E).
The haplotypes distribution and significant association were clarified in Figure 1, XPD have high percentage of di haplotype than control group in significant association (X2 9.55152, p=0.0019), while uni haplotype is low frequent in cases than control group.
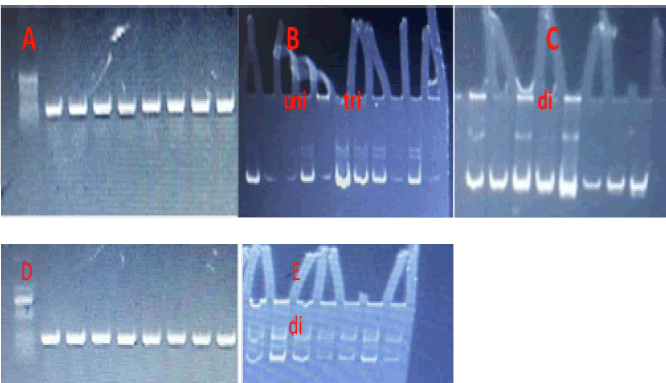
Figure 1: Electrophoresis patterns of DNA repair genes, (A and D) XPD and hOGG amplifications products using agaros gell (1% agaros, 100 V for 30 min), (B and C) hOGG electrophoresis using PCRSSCP technique with three haplotypes, (E) electrophoresis using PCR-SSCP technique of XPD with di haplotype
The hOGG haplotype distribution in study groups show significant association of di haplotype with cases than control in addition to disappear of uni haplotype in cases (X2 28.8245, P0.000) (Figure 2).
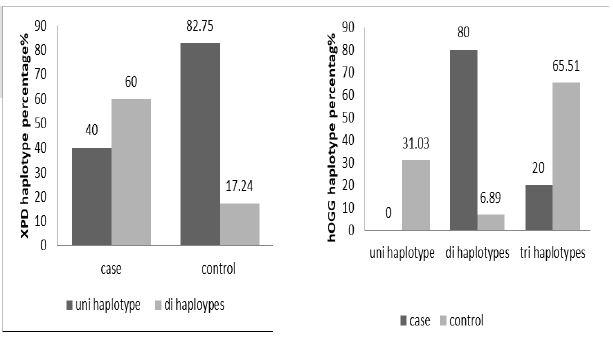
Figure 2: The haplotypes percentage distribution of XPD (X2 9.55152, p 0.0019) and hOGG gene in study groups (X2 28.8245, P0.000)
The genes haplotypes were distributed according work time in cases per day to more and less than 6 hours, XPD showed nonsignificant association XPD (X2= 0.00576, p=0.9395), uni and di haplotype more frequent in cases exposure to x-ray less than 6 hours per day (Figure 2). The hOGG appeared in significant association with work time (X2 8.14815, p 0.000) that more frequent of di haplotype in less than 6 hours category (Figure 3).
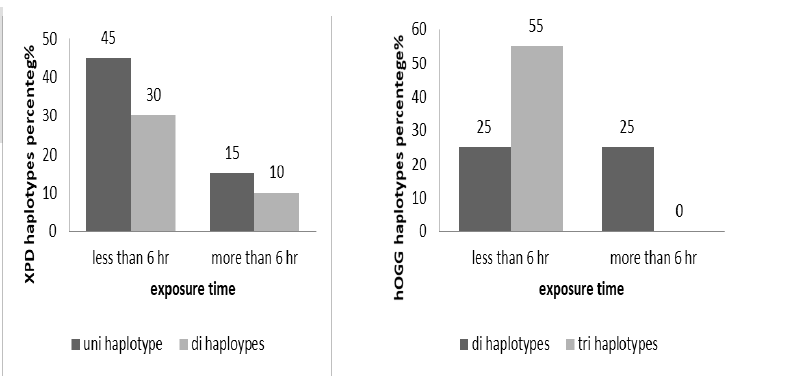
Figure 3: The haplotypes percentage distribution of XPD(X2= 0.00576, p=0.9395) and hOGG (X2 8.14815, p 0.000) according to exposure time (less and more than 6 hours)
The work time of technicians classified to more and less than 6 hours per day, the haplotypes distribution clarified in Figure 4, XPD non-significant association with work time (X2=0.6694, p=0.4132), di haplotype more frequent in case work less than 6 hours per day. The di haplotypes more frequent in worker with less than 6 hours per day in non-significant association (X2=0.6694, p= 0.4132) (Figure 4).
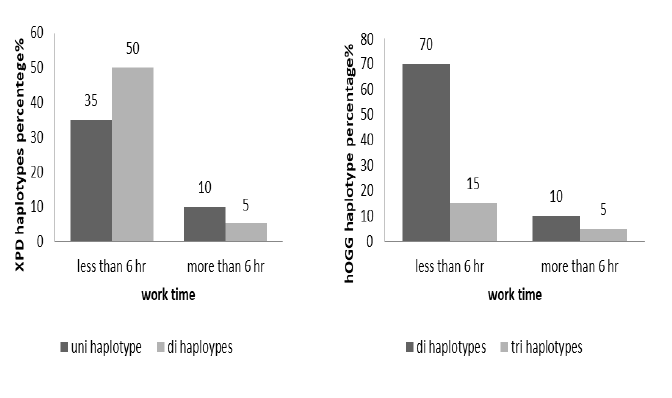
Figure 4: The haplotypes percentage distribution of XPD(X2= 0.6694, p 0.4132) and hOGG (X2 0.392, p 0.5312) according to work time per day (less and more than 6 hours)
The exposure day to x-ray classified to less and more than 4 days per week, XPD non-significant association with exposure day (X2 0.0653, p=0.798) about 50% of worker have uni haplotype that work less than 4 days. The hOGG haplotypes also non-significant association with exposure day (X2 0.0653, p=0.798) in spite of 70% of workers have di haplotype (Figure 5).
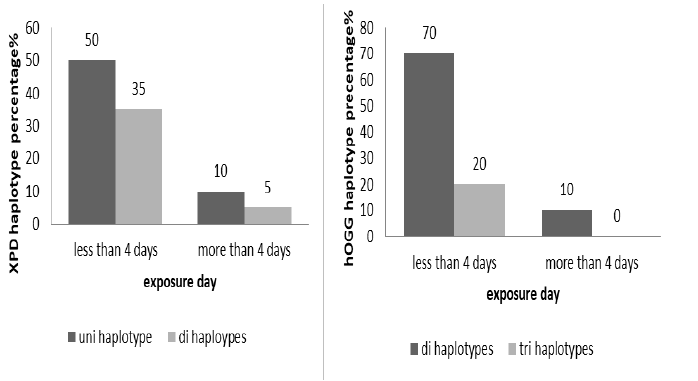
Figure 5: The haplotypes percentage distribution of XPD (X2 0.0653, p 0.798) and hOGG (X2 0.5555, p 0.4561) according to exposure day number (less and more than 4 days)
Different work period were reported and classified to less and more than 10 years, XPD showed non-significant association (X2 0.3030, p=0.582) with work period. Uni haplotype was frequent in same percentage in both categories. The hOGG also non-significant correlations with work periods (X2=1.8181, p=0.1775), di haplotype more frequent in worker with less than 10 years (Figure 6).
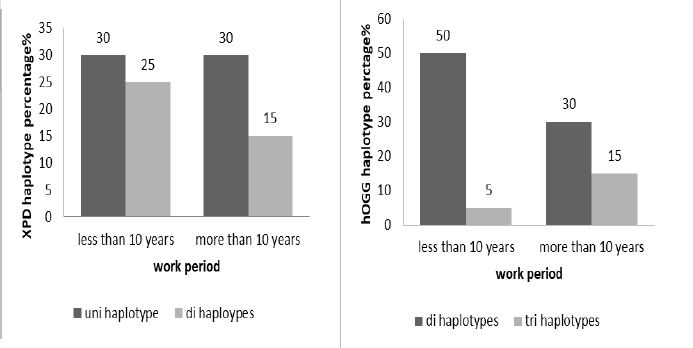
Figure 6: he haplotypes percentage distribution of XPD(X2 0.3030, p 0.582) and hOGG (X2 1.8181, p 0.1775) according to work period (less and more than 10 years)
Discussion
The work in medication departments in some Iraqi hospitals have been observed to be without Health standards and laws. Thus varies harmful effects may implicated in disease incidence, the current study was suggested to estimate important genes that involved in DNA repair. The effect of X-ray in the cell components has been studied. Ann Christin [19] suggested that to improve radiotherapy, radiosensitivity in S phase could be increased by combining irradiation with agents that induce secondary DSB or inhibit checkpoint signalling.
Some reports describe the X-ray irradiation effects on DNA molecules in biological conditions, especially in medical applications, DNA strand breaks, Mutations, structural changes such as gene order rearrangements and chemical modifications of bases have been identified [20,21,22]. Diffusion and Creation of radicals during X-ray exposure have been associated to modifications in molecular structure [23, 24]. The strand break may be caused by X-ray photons [25]. In organisms, the repair mechanisms found to be withstand moderate levels of radiationinduced lesions [26]. The mutation in DNA may be existed in repair genes as observed in current study that lead to absence of repair mechanisms, the accumulated of damages with increasing X-ray dose a new scan is adding to the impacts of the previous ones. When molecules with DNA alterations like oxidative and hydrolytic lesions as a result of natural decay and taphonomic mechanisms [27, 28]. The analysis of downstream genetic can be affected in cases have X-ray dose accumulation with significant levels. Particularly the hydrolytic conditions seem to have a major role as demonstrated in a report on simulated impacts of X-radiation on fragmented DNA in different conditions as well as wet, dry and frozen states, the radiation-induced DNA damage highest probability occurs in a wet state [29].
On the other hand, Wang et al., supposed that the effects of x-ray depended on the exposure dose and type of x-ray [30]. The safe dose should be very low dose of KV X-rays, the Mega-V-X-rays have been found to be dose-dependent. The higher dose than the damage threshold would be harmful, that between (1.0 - 1.5 Gray) may have some benefits like cell growth and gene transfer.
The Radiation stimulate different DNA lesions, about 10,000 bases were damaged, about 1,000 single strand breaks and 40 double strand breaking that stimulate during exposure to a gray per cell [31, 32]. These damages if didn’t corrected it may be caused cell death by mitotic catastrophe and apoptosis. The double strand breaks is more harmful that can trigger cell death [33].
The association between x-ray and DNA repair genes was studied in some reports like Hu et al., found that amino acid substitution variants of XRCC1 and APE1 may contribute to IR hypersensitivity [34]. Toprani et al., observed that the base excision repair gene polymorphisms including (hOGG1, APE1, XRCC1, and LIGASE1 ) play important role in identifying donors with radio-sensitivity and Radio-adaptive response in human cells [35].
The deficiency in DNA repair mechanisms lead to different diseases and the result of present study is an important to take in consideration in medication department employees that work without health awareness about protective tools used during work to avoid harmful effects.
Conclusion
The exposure time, exposure day, work time and work period didn’t have effects in the hOGG and XPD haplotypes but strong association with x-ray technicians. This mean that there were vital role of x-ray in human genome in addition to decline in the hOGG Ser326Cys and (Arg399Gln) XPD.
References
- von Sonntag C. The chemical basis of radiation biology. 1987.
- Olive PL, Banáth JP. Phosphorylation of histone H2AX as a measure of radiosensitivity. Int J Radiat Oncol Biol Phys. 2004;58:331-335.
- Frankenberg-Schwager M, Frankenberg D, Harbich R. Different oxygen enhancement ratios for induced and unrejoined DNA double-strand breaks in eukaryotic cells. Radiat. res. 1991;128(3):243-50.
- Schulhof JC, Molko D, Teoule R. Synthesis of DNA fragments containing 5, 6-dihydrothymine, a major product of thymine gamma radiolysis. Nucleic acids res.1988;16:319-326.
- Lesiak KB, Wheeler KT. Formation of α-deoxyadenosine in polydeoxynucleotides exposed to ionizing radiation under anoxic conditions. Radiat. res.. 1990;121:328-337.
- Dizdaroglu M, Jaruga P, Rodriguez H. Identification and quantification of 8, 5′-cyclo-2′-deoxy-adenosine in DNA by liquid chromatography/mass spectrometry. Free Radic. Biol. Med.2001;30:774-784.
- Shoulkamy MI, Nakano T, Ohshima M, Hirayama R, Uzawa A, et al. Detection of DNA–protein crosslinks (DPCs) by novel direct fluorescence labeling methods: distinct stabilities of aldehyde and radiation-induced DPCs. Nucleic acids res. 2012;40:143-.
- Nakano T, Mitsusada Y, Salem AM, Shoulkamy MI, Sugimoto T ,et al. Induction of DNA-protein cross-links by ionizing radiation and their elimination from the genome. Mutat Res 2015;771:45–50.
- Lehmann AR. The xeroderma pigmentosum group D (XPD) gene: one gene, two functions, three diseases. Genes dev..2001;15:15-23.
- Hou SM, Fält S, Angelini S, Yang K, Nyberg F, et al. The XPD variant alleles are associated with increased aromatic DNA adduct level and lung cancer risk. carcinogenesis. 2002;23:599-603..
- Matullo G, Palli D, Peluso M, Guarrera S, Carturan S,et al. XRCC1, XRCC3, XPD gene polymorphisms, smoking and 32 P-DNA adducts in a sample of healthy subjects. Carcinogenesis. 2001;22:1437-1445.
- Palli D, Russo A, Masala G, Saieva C, Guarrera S,et al. DNA adduct levels and DNA repair polymorphisms in trafficâ?exposed workers and a general population sample. Int. J. Cancer.2001 ;94:121-127.
- Spitz MR, Wu X, Wang Y, Wang LE, Shete S,et al .Modulation of nucleotide excision repair capacity by XPD polymorphisms in lung cancer patients. Cancer res. 2001;61:1354-1357.
- Ma L, Chu H, Wang M, Shi D, Zhong D, et al.hOGG 1 S er326 C ys polymorphism is associated with risk of bladder cancer in a C hinese population: A caseâ?control study. Cancer sci. 2012;103:1215-1220.
- Boiteux S, Radicella JP. The human OGG1 gene: structure, functions, and its implication in the process of carcinogenesis. arch. biochem. biophys. . 2000;377:1-8.
- Yamane A, Kohno T, Ito K et al Differential ability of polymorphic OGG1 proteins to suppress mutagenesis induced by 8â?hydroxyguanine in human cell in vivo. Carcinogenesis.2004;25:1689–1694.
- Kohno T, Shinmura K, Tosaka M, Tani M, Kim SR, et al. Genetic polymorphisms and alternative splicing of the hOGG 1 gene, that is involved in the repair of 8â?hydroxyguanine in damaged DNA. Oncogene.1998;16:3219–25.
- Das S, Purkayastha S, Roy H, Sinha A, Choudhury Y. Polymorphisms in DNA repair genes increase the risk for type 2 diabetes mellitus and hypertension. Biomol Concepts.2018;9:80-93.
[Google Scholar] [ Cross Ref]
- Parplys AC, Petermann E, Petersen C, Dikomey E, Borgmann K. DNA damage by X-rays and their impact on replication processes. Radiother Oncol. 2012;102:466-471.
[Google Scholar] [ Cross Ref]
- Wolff S. Radiation Genetics. Annu Rev Genet. 1967;1:221-224.
[Google Scholar] [ Cross Ref]
- Liber HL, Leong PM, Terry VH, Little JB. X-rays mutate human lymphoblast cells at genetic loci that should respond only to point mutagens. Mutat Res. 1986;163:91-97.
[Google Scholar] [ Cross Ref]
- Grosovky AJ, de Boer JG, de Jong PJ, Drobetsky EA, Glickman BW. Base substitutions, frameshifts, and small deletions constitute ionizing radiation-induced point mutations in mammalian cells. Proc Natl Acad Sci USA. 1988;85:185-188.
[Google Scholar] [ Cross Ref]
- Jefferies K. In Introduction to Radiography and Patient Care (eds Adler AM, Carlton RR), Ch. 9. WB Saunders Company; 1999.
[Google Scholar] [ Cross Ref]
- Bushong SC. Radiologic Science for Technologists: Physics, Biology and Protection. Mosby; 2001.
[Google Scholar ] [Cross Ref]
- Roots R, Okada S. Estimation of lifetimes and diffusion distances of radicals involved in x-ray-induced DNA strand breaks of killing of mammalian cells. Radiat Res. 1975;64:306-320.
[Google Scholar] [ Cross Ref]
- Clancy S. DNA damage & repair: mechanisms for maintaining DNA integrity. Nat. Educ.. 2008;1.
- Lindahl T. Instability and decay of the primary structure of DNA. Nat. 1993;362:709-715.
- Mitchell D, Willerslev E, Hansen A. Damage and repair of ancient DNA. Mutat Res. 2005;571:265-276.
[Google Scholar] [ Cross Ref]
- Wanek J, Ruhli FJ. Risk to fragmented DNA in dry, wet, and frozen states from computed tomography: a comparative theoretical study. Radiat Env. Biophys,2016.
[Google Scholar] [ Cross Ref]
- Wang Z, Lv MY, Huang YX. Effects of Low-Dose X-Ray on Cell Growth, Membrane Permeability, DNA Damage and Gene Transfer Efficiency. Dose Response. 2020;18.
[Google Scholar] [ Cross Ref]
- Ward JF. DNA damage and repair. Basic Life Sci. 1991;58:403-415.
[Google Scholar] [ Cross Ref]
- Burkart W, Jung T, Frasch G. Damage pattern as a function of radiation quality and other factors. C R Acad Sci III. 1999;322:89-101.
[Google Scholar] [ Cross Ref]
- Radford IR. The level of induced DNA double-strand breakage correlates with cell killing after X-irradiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1985;48:45-54.
- Hu JJ, Smith TR, Miller MS, Mohrenweiser HW, Golden A, et al. Amino acid substitution variants of APE1 and XRCC1 genes associated with ionizing radiation sensitivity. Carcinogenesis. 2001;22:917-922.
[Google Scholar ] [ Cross Ref]
- Toprani SM, Das B. Radio-adaptive response, individual radio-sensitivity and correlation of base excision repair gene polymorphism (hOGG 1, APE1, XRCC1, and LIGASE1) in human peripheral blood mononuclear cells exposed to gamma radiation. Environ Mol Mutagen.2020;61:551-559.
[Google Scholar ] [ Cross Ref]

