Research Article - Onkologia i Radioterapia ( 2022) Volume 16, Issue 12
The treatment of non-invasive bladder tumours with transurethral resection and intravesical instillation of Mitomycin C
Nuraj Petrit1*, Gllareva Bashkim1 and Beqiri Agron1Nuraj Petrit, Department of Urology , University Clinical Center of Kosovo, Kosovo, Email: petrit.nuraj@uni-pr.edu
Received: 08-Dec-2022, Manuscript No. OAR-22-82894; Accepted: 20-Dec-2022, Pre QC No. OAR-22-82894 (PQ); Editor assigned: 09-Dec-2022, Pre QC No. OAR-22-82894 (PQ); Reviewed: 14-Dec-2022, QC No. OAR-22-82894 (Q); Revised: 16-Dec-2022, Manuscript No. OAR-22-82894 (R); Published: 23-Dec-2022
Abstract
Background: Approximately 75%-80% of the cases with bladder cancers are discovered when the tumor is located on the lamina mucosa (Ta, Tis), or on the lamina propria (T1). Whilst 15%-25 % of bladder cancers are discovered when the tumour has invasive evolution and has evolved also the muscle extent and on, or has given metastases on the lymphatic glands.
Objective: Is it effective Mitomycin in the treatment of non-invasive bladder tumours Ta-T1 after transurethral resection.
Material and Methods: The research was conducted with patients of the Urology Clinic, University Clinical Centre of Kosovo, in Prishtina. The study included 108 patients with non-invasive bladder tumours Ta T1. After TURBT, we applied intravesical instillation Mitomycin C 40 mg within the first 6 hours of the transurethral resection. After the histopathological result, a week after the TURBT, we instilled Mitomycin C once a week for 6 weeks, then once a month for 2 months. The follow up was 36 months. From the statistical parameters, the structure index, arithmetic mean, and standard deviation were calculated. Qualitative data testing was done with X2 test.
Results: The study included 108 patients with bladder tumours of which 72.2% were male and 27.8% were female. X2-test showed statistically significant difference of cases by gender (X2=11.2, P=0.001). The age (mean± SD) was 63.1 ± 11.3 years. Current smokers were 63% of cases. Tumours grade were: PUNLMP 19.4%, low grade 58.3%, high grade 22.2% of cases. X2- test showed statistically significant difference (X2=11.2, P=0.001). Multifocality with tumours were 76.9% of solitary and 23.1% of multiple tumours. The tumours recurred in 41 patients (37.9%). Morphologically, of which 75% were defined with tumour size <3 cm and 25% with tumour size >3 cm. The tumour stage was as Ta 43.5% and T1 56.5% of cases.
Conclusion: The effective therapy for non-invasive bladder tumours (Ta-T1) is TURBT with intravesical instillation of Mitomycin C. Diagnosing these tumours at their early stages is the key for the best treatment and prognoses. Intravesical chemotherapy is safe and tolerable for non-invasive bladder tumours and has reduced tumour recurrence rates, especially when the grade is low.
Keywords
bladder tumours; mitomycin c; transurethral resection; stage; grade
Introduction
At diagnosis, 75%-80 % of bladder tumors are Non-Muscle Invasive (NMIBC) and confined to the urothelium and/or lamina propria. These include papillary tumors, Ta (confined to urothelium) and T1 (lamina propria invasion) or carcinoma in situ (CIS), a flat erythematous lesion [1, 2]. A Transurethral Resection of the Bladder Tumour (TURBT) is the standard treatment for Ta and T1 bladder tumours and helps in establishing the diagnosis, staging and assigning a risk profile [3, 4]. For low-grade papillary (pTaG1) tumours TURBT may be the only treatment required. However, tumour recurrence is a major problem with higher grade Ta and T1 tumours. At 1 year following TURBT about 20% of patients with low-risk NMIBC and 40% of those with medium-risk NMIBC will develop tumour recurrence. Patients with high-risk NMIBC will express an even higher recurrence rate (90%) at 1 years-2 years following TURBT [5]. The management of non-muscle invasive bladder cancer has become more complex with regard to initial investigation, treatment and follow up [6]. Smoking leads to higher mortality from bladder cancer during long-term follow up, even though in a multivariate analysis the prognostic effect of smoking was weaker than that of other factors, such as stage, grade, size and multifocality of the tumour [7]. Bladder cancer is also associated with industrial exposure to aromatic amines in dyes, paints, solvents, leather dust, inks, combustion products, rubber, and textiles.
Approximately 80%-90% of patients with bladder cancer present with painless gross haematuria, which is the classic presentation, but 20%–30% of patients with bladder cancer experience irritative bladder symptoms [8]. Intravesical therapy can also be given as a maintenance therapy as opposed to an induction course alone to provide long-term immune-stimulation or local chemotoxicity aimed at preventing tumour recurrence. The objective of intravesical chemotherapy is to eradicate microscopic residual tumour, prevent tumour recurrence and progression.
The diagnosis of bladder cancer ultimately depends on cystoscopic examination of the bladder and histological evaluation of the resected tissue. Small tumours can be resected in one chip where the chip contains the complete tumour plus a part of the underlying bladder wall. Larger tumours have to be resected in fractions. Although a state-of-the-art Transurethral Resection (TUR) by itself could eradicate a Ta or T1 tumour completely, these tumours recur in a high percentage of patients and progress to muscle-invasive bladder cancer in a significant number of cases. The choice between chemotherapy and immunotherapy largely depends on the risk that needs to be reduced recurrence or progression [9].
Materials and Methods
The research was conducted on our patients at the Urology Clinic, University Clinical Centre of Kosovo, in Pristine. The study was prospective and patients are of both genders. The study protocol was approved by the Ethics Committee of the Faculty of Medicine in Pristine. The study included 108 patients with non-invasive bladder tumours. The diagnosing methods were: the anamnesis, biochemical laboratory examinations, echosonography, cystoscopy, CT, transurethral resection and hystopathological examination All patients before participating in the study were informed about our research, and after receiving their consent we started the research. The follow up time was 36 months. The inclusion criteria were as follows: patients with non-invasive transitional cell carcinoma of the bladder who were managed with transurethral resection and induction and maintenance therapy with intravesical doxorubicin.
After the diagnosis was determined by cystoscopy, the patients were admitted to the Department of Urology and prepared for transurethral resection of bladder tumour (TUR-BT). During the procedure, the tumour characteristics in terms of size (≤ 3 cm, >3 cm) and number (solitary, multiple) were recorded. Intravesical chemotherapy was given routinely to patients with superficial bladder carcinoma Ta-T1 (non-invasive bladder tumours). We included only the patients with Transitional Cell Carcinoma (TCC); we diagnosed one case with adenocarcinoma, but it was excluded. All patients consented to the adjuvant therapy. After TURBT (transurethral resection bladder tumour), in all of the cases we applied intravesical therapy (Mitomycin C 40 mg and 40 ml normal saline), within the first 6 hours of the surgery (apart from those cases that were bleeding). This therapy has lasted for two hours in the bladder, and the further adjuvant treatment was according to the histopathological results.
After the histopathological result, a week after the TURBT, we continue session of mitomycin was administrated. Each session began by emptying the bladder of any residual urine by a urethral catheter. Then we instilled 40 mg of mitomycin diluted with 40 ml normal saline, and removed the catheter. We asked the patient to rest in a bed for 110 minutes by taking right lateral, left lateral, prone and supine positions, and then walking in the hall for another 10 minutes before voiding out the drug. The session was repeated once a week for 6 weeks, then once a month for 2 months. We further excluded any patient who was not given this regimen or was not given the maintenance therapy. The rationale for the selection of mitomycin was its local availability. Mitomycin also represents an important drug for adjuvant intravesical chemotherapy. Although BCG is considered the first choice as intravesical adjuvant therapy, it is not always available in the present setting. Secondly, BCG is not relevant for most patients in our study since we had no cases of CIS.
Mainly, we followed up the patients by using cystoscopy and urinary cytology every 3 months in the first two years and every 6 months after that. We also performed a cystoscopy when patients had documented haematuria.
Statistical analysis
The obtained data were presented through tables and figure. From the statistical parameters the structure index, arithmetic mean, and standard deviation calculated. Qualitative data testing was done with X2-test or Fisher test.
Results
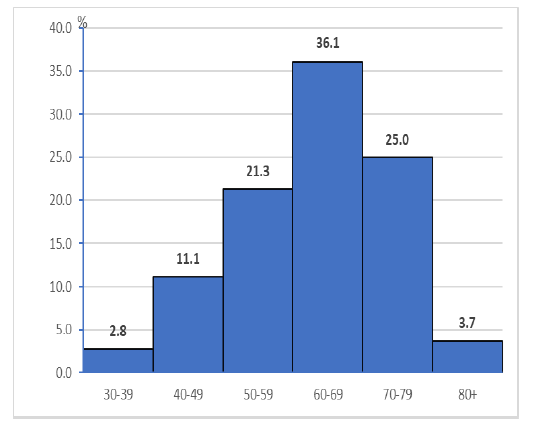
According to the age group out of 108 patients included in the research; 3 (2.8%) are in the age group 30 years-39 years, 12 (11.1%) are in the age group 40 years-49 years, 23 (21.3%) are of age group 50 years-59 years, 39 (36.1%) are in the age group 60 years-69 years, 27 (25%) are in the age group 70 years-79 years, and 4 (3.7%) are in the age group 80 and more.
The age (mean ± SD) was 63.1 ± 11.3 years, and the peak incidence was between 60 years-69 years (Figure 1). The study included 108 patients with bladder tumours of which 78 (72.2%) were male and 30 (27.8%) were female. X2-test showed statistically significant difference in the number of cases by gender (X2=11.2,P= 0.001), (Table.1).
Figure 1: Structure of patients by age group
Tab.1. Patients characteristics
| Characteristics | N | % | X2 | p-value |
|---|---|---|---|---|
| Gender | 108 | 100 | ||
| Male | 78 | 72.2 | 11.22 | 0.001 |
| Female | 30 | 27.8 | ||
| Age(mean±SD) | 63.1±11.3 | |||
|
Smoking history |
||||
| Never smoker | 8 | 7.4 | ||
| Former smoker | 32 | 29.6 | ||
| Current smoker | 68 | 63 | 31.29 | 0 |
Smoking was the common risk factor for developing bladder cancer among our patients. Most patients were heavy current smokers 68 (63%) of cases, defined as smoking more than 1 pack per day for 30 years or more, former smoker 32 (29.6%) defined as smoking less than 1 pack/day for 20 years, and never smoker 8 (7.4%). X2- test showed statistically significant difference in the number of cases by current smokers than former smokers (X2=31.2, P=0.000), (Table1). The follow up of the patients continued successfully for up to 36 months.
Morphologically, of which 81 (75%) were defined with tumour size <3 cm and 27 (25%) of cases with tumour size >3 cm. X2- test showed statistically significant difference in the number of cases by tumours size (X2=14.4, P= 0.000).
In our research the tumor stage were as Ta (Non-invasive papillary carcinoma) 43.5% of cases and T1 (Tumour invades subepithelial connective tissue) 56.5% of cases.
Tumour grade in our study were: PUNLMP (Papillary urothelial neoplasm of low malignant potential) were 21 (19.4%) of cases, low grade tumours were the predominant type with 63 (58.3%) of cases, followed by high grade with 24 (22.2%) of cases. X2- test showed statistically significant difference in the number of cases by low grade tumours than PUNLMP and high grade tumours (X2=11.2, P=0.001). Multifocality of cases with tumours were 83 (76.9%) of solitary tumours and 25 (23.1%) of multiple tumours. ReTURBT is done at 42 (38.9%) of patients. The tumours recurred in 41 patients (37.9%), (Table 2).
Tab. 2. Characteristics of urinary bladder tumours
| N | % | X2 | p-value | ||
|---|---|---|---|---|---|
| Tumor size (cm) | 108 | 100 | |||
| <3 cm | 81 | 75 | 14.4 | 0 | |
| >3 cm | 27 | 25 | |||
| Tumor Staging | Ta | 47 | 43.5 | 0.91 | 0.034 |
| T1 | 61 | 56.5 | |||
| Tumor grade | PUNLMP | 21 | 19.4 | 13.71 | 0.001 |
| Low grade | 63 | 58.3 | |||
| High grade | 24 | 22.2 | |||
| Multifocality | Solitary | 83 | 76.9 | 16.78 | 0 |
| Multiple | 25 | 23.1 | |||
| Re TURBT | Yes | 42 | 38.9 | 2.7 | 0.1 |
| No | 66 | 61.1 | |||
| Nr. of tumor recurrence | In 1st year | 8 | 7.4 | 2.68 | 0.262 |
| In 2nd year | 13 | 12 | |||
| in 3 rd year | 20 | 18.5 |
Adverse drug reactions after instillation of Mitomycin seen during the session or during follow up. The irritative voiding symptoms were the most common side effects; urinary frequency (20.4 %) of cases, dysuria (15.7%), hematuria (13%), urinary tract infection (8.3%), gastrointestinal reaction (2.8%) of cases. X2- test showed statistically significant difference in the number of cases by adverse drug reactions (X2=9.86, P=0.042), (Table 3).
Tab. 3. Adverse drug reactions after intravesical instillation of Doxorubicin
| Adverse reactions | N | % |
|---|---|---|
| 108 | 100 | |
| Urinary frequency | 22 | 20.4 |
| Dysuria | 17 | 15.7 |
| Haematuria | 14 | 13 |
| Urinary tract infection | 9 | 8.3 |
| Gastrointestinal reaction | 3 | 2.8 |
| X2 = 9.86 | p = 0.042 | |
Discussion
The diagnosis of bladder cancer ultimately depends on cystoscopic examination of the bladder and histological evaluation of the resected tissue. Mitomicin intravesical administration mainly affects the recurrence rate; therefore, we concentrated on evaluating the disease recurrence as well as side effects, complications and patients tolerance of the instillation. Males were more dominant in this study compared to other studies. This could be explained by the low incidence of smoking in women, and the fact that women rarely deal with the carcinogenic agents [10].
In our study included 108 patients with bladder tumours of which 78 (72.2%) were male and 30 (27.8%) were female. X2-test showed statistically significant difference in the number of cases by gender (X2=11.2, P=0.001). The age (mean ± SD) was 63.1 ±11.3 years, and the peak incidence was between 60 - 69 years.
Smoking was the common risk factor for developing bladder cancer among our patients. Most patients were heavy current smokers 68 (63%) of cases. The follow-up of the patients continued successfully for up to 36 months.
In patients at low risk of tumor recurrence and progression immediate instillation of single dose of chemotherapy is recommended as the adjuvant treatment. In patients at intermediate or high risk of recurrence, one immediate instillation of chemotherapy followed by further instillations of chemotherapy for a minimum of 1 year [11-13]. The time period within which the installation is completed is very important. In all the studies included in the EORTC meta-analysis, the instillation was administered within 24 hour Kaasinen et al, reported that the risk of recurrence is twice when the instillation was not given within 24 hour of TURBT [14].
In our research, after TURBT (transurethral resection bladder tumour), in all of the cases we applied intravesical therapy (Mitomycin C 40 mg and 40 ml normal saline), within the first 6 hours of the surgery (apart from those cases that were bleeding). T his therapy has lasted for two hours in the bladder, and the further adjuvant treatment was according to the histopathological results. After the histopathological result, a week after the TURBT, we continue session of mitomicin was administrated.
As to the grade, we notice that the grade of tumour is an important indicator of recurrence: more than half of the patients with high (56%) had recurrence during follow-up, but none with PUNLMP. This accords with the opinion that high grade is a relative indication for radical cystectomy [15]. The size and number of tumours also are shown; they are important indicators of recurrence, but less important than the grade. A total of 41% of patients with tumours greater than 2.5cm recurred and 44% of patients with multiple tumours recurred.
Tumour grade in our study were: PUNLMP (Papillary urothelial neoplasm of low malignant potential) were 21 (19.4%) of cases, low grade tumours were the predominant type with 63 (58.3%) of cases, followed by high grade with 24 (22.2%) of cases. Multifocality of cases with tumours were 83 (76.9%) of solitary tumours and 25 (23.1%) of multiple tumours.
Saika et al. noted that only the adjuvant therapy (chemotherapy or immunotherapy) played an important role in the recurrence rate, when the non-invasive tumours were high grade, while the size, multifocality and morphology did not play a role in terms of the recurrence rate [15]. This difference to our results is perhaps unreal and could be occasioned by the fact that the tumours were high grade. Cheng et al. showed in long term follow-up (17 years) that adjuvant intravesical mitomycin did not improve the recurrence rate of superficial bladder cancer, compared with controls on long-term follow-up. Tumour size and grade were shown to be prognostic factors for recurrence and progression respectively, therefore we acknowledge the importance of long term follow-up in order to get valid conclusions [16].
In our study morphologically, of which 81 (75%) were defined with tumour size <3 cm and 27 (25%) of cases with tumour size >3 cm. X2-test showed statistically significant difference in the number of cases by tumours size (X2=14.4, P=0.000).
A meta-analysis of European Organization for Research and Treatment of Cancer (EORTC) and Medical Research Council (MRC) data, comparing intravesical chemotherapy versus TUR alone, demonstrated that chemotherapy does prevent recurrence but not progression. There is no one superior drug with regard to efficacy. Mitomycin C, epirubicin and doxorubicin have all been shown to have a beneficial effect [17]. A randomised trial documented that the concentration of the drug instilled was more important than the duration of the treatment [18].
In our study the tumor stage were as Ta (Non-invasive papillary carcinoma) 43.5% of cases and T1 (Tumour invades subepithelial connective tissue) 56.5% of cases. The tumours recurred in 41 patients (37.9%).
T he probability for recurrence and progression at 1 year vary from 15% to 61% and 0.2% to 17%, respectively. After 5 years of followup, recurrence and progression rates range from 31% to 78% and 0.8% – 45% respectively [19-23].
Conclusion
The effective therapy for Non-invasive bladder tumours (Ta-T1) is TURBT with intravesical Mitomycin C. Diagnosing these tumours at their early stages is the key for the best treatment and prognoses. Intravesical chemotherapy is safe and tolerable for non-invasive bladder tumours and has reduced tumour recurrence rates, especially when the grade is low. After TUR and the adjuvant therapy it is of an eminent importance the further examination with cystoscopy and urinary cytology. In cases of often recidivism, those with multifocal localization, with high level of malignancy, the radical cystectomy with urinary derivation should be applied. T he grade, stage, size, and multifocality of tumour are important prognostic indicators.With minimal side effects, intravesical instillation of mitomycin could be used as one of the interventions for non-invasive bladder tumours.
Conflict of Interest
The authors declare no conflict of interest or financial.
Funding
None
Author Contributions
All authors contributed to all stages of drafting this paper and, gave final approval of the version to be published.
References
- Peyronnet B, Seisen T, Dominguez-Escrig JL, Bruins HM, Yuan CY, Lam T,et al. Oncological outcomes of laparoscopic nephroureterectomy versus open radical nephroureterectomy for upper tract urothelial carcinoma: an European Association of Urology guidelines systematic review. Eur urol focus. 2019;5:205-23
- Babjuk M , Bohle A , Burger M, Capoun O, Cohen D,et alEAU Guidelines on Non-Muscle-Invasive Urothelial Carcinoma of the Bladder Eur Urol, 2017.71:447-61
- O’Donnell MA. Practical applications of intravesical chemotherapy and immunotherapy in high-risk patients with superficial bladder cancer. Urol Clin North Am. 2005;32:121-31.
- Adiyat KT, Katkoori D, Soloway CT, De los Santos R, Manoharan M,et al. Complete transurethral resection of bladder tumor: Are the guidelines being followed? Urology.2010;75:365-367.
- Sylvester RJ, Oosterlinck W, van der Meijden AP. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: A meta-analysis of published results of randomized clinical trials.J Urol. 2004;171:2186-90.
- Oosterlinck W, Lobel B, Malnstrom PU, Stockle M, Sternberg C.Guidelines on bladder cancer. Eur Urol.2002;24:105-112.
- Aveyard F, Adab F, Cheng K, Wallace D, Hey K, Murphy M. Does smoking status influence the prognosis of bladder cancer? A systematic review.BJU Int. 2002;90:228-239.
- Grossfeld GD, Litwin MS, Wolf JS. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy part II: patient evaluation, cytology, voided markers, imaging, cystoscopy, nephrology evaluation, and follow-up.Urology.2001;57:604 -610.
- Han RF, Pan JG. Can intravesical bacillus Calmette-Guerin reduce recurrence in patients with superficial bladder cancer? A meta-analysis of randomized trials.Urology.2006;67:1216-1223.
- Messing EM. Urothelial tumors of the bladder.Cambell-Walsh Ulorogy. 2007.
- Babjuk M, Oosterlinck W, Sylvester R, Kaasinen E, Bohle A,et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder.Eur Urol.2008;54:303–314.
- Parkin DM.The global burden of urinary bladder cancer. Scand J Urol Nephrol Suppl.2008;48:12-20.
- Hall MC, Chang SS, Dalbagni G, Pruthi RS, Seigne JD,et al.Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol. 2007; 178:2314–2330.
- Kaasinen E, Rintala E, Hellström P, Viitanen J, Juusela H, et al. Factors explaining recurrence in patients undergoing chemoimmunotherapy regimens for frequently recurring superficialbladdercarcinoma. Eur Urol. 2002; 42:167-174.
- Saika T, Tsushima T. Clinical study of G3 superficial bladder cancer without CIS treated with conservative therapy.Jpn J Clin Oncol. 2002;11:461-465.
- Cheng C, Chan P, Chan L, Chan C, Ng C, Lai M. 17-year follow-up of a randomized prospective controlled trial of adjuvant intravesical doxorubicin in the treatment of superficial bladder cancer. Int Braz J Urol. 2005;31:204–213.
- Buckland G, Ros MM, Roswall N, Bueno‐de‐Mesquita HB, Travier N,et al. Adherence to the Mediterranean diet and risk of bladder cancer in the EPIC cohort study. International journal of cancer. 2014;134:2504-2511
- Burger M, Catto J, Dalbagni G, H. Barton Grossman H,et al.Epidemiology and risk factors of urothelial bladder cancer. Eur Urol, 2013.63:234-241
- Kuroda M, Niijima T, Kotake T, Akaza H, Hinotsu S. Effect of Prophylactic Treatment with Intravesical Epirubicin on Recurrence of Superficial Bladder Cancer—The 6th Trial of the Japanese Urological Cancer Research Group (JUCRG):: A Randomized Trial of Intravesical Epirubicin at Dose of 20 mg/40 ml, 30 mg/40 ml, 40 mg/40 ml. Eur Urol. 2004;45:600-605.
- Sylvester RJ, vail der,Meijden AP, Oosterlinck W, Witjes A, et al. Predicting recurrence and progression in individual patients with stage TaT1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven FORTC trials. Eur Urol. 2006; 48:466-477.
- Bohle A, Bock P. Intravesical bacillus Calmette-Guerin versus mitomycin C in superficial bladder cancer: formal meta-analysis of comparative studies on tumour progression.Urology.2004;63:682-686.
- Malmstrom PU, Sylvester RJ, Crawford DE, Friedrich M, Krege S,et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guerin for non-muscle-invasive bladder cancer.Eur Urol. 2009;56:247-256.
- Brandau S, Suttmann H. Thirty years of BCG immunotherapy for non-muscle invasive bladder cancer: A success story with room for improvement.Biomed Pharmaco ther. 2007;61:299-305.