Original Research Article - Onkologia i Radioterapia ( 2023) Volume 17, Issue 3
Therapeutic outcome of Differentiated Thyroid Carcinoma
Mohammed H. Alqambar*, Jamal Alsaeed, Abdulaziz Alwosabie, Njoud Alkaldi, Amal Abu alsaud, Zainab Alsaffar, Bashayer Aldossary, Gadeer Emsaad, Sahar Alradwan, Omar Amin, Mansour Alenezy, Mais Saradi and Fatima KalifaMohammed H. Alqambar, King Fahad Specialist Hospital, Dammam, Saudi Arabia, Email: H.qambar@kfsh.med.sa
Received: 20-Feb-2023, Manuscript No. OAR-23-89680; Accepted: 15-Mar-2023, Pre QC No. OAR-23-89680 (PQ); Editor assigned: 22-Feb-2023, Pre QC No. OAR-23-89680 (PQ); Reviewed: 08-Mar-2023, QC No. OAR-23-89680 (Q); Revised: 10-Mar-2023, Manuscript No. OAR-23-89680 (R); Published: 17-Mar-2023
Abstract
Introduction: The majority of patients with Differentiated Thyroid Cancer (DTC) diagnosis have long-term survival, a considerable number may have a persistent or recurrent illness, and some may ultimately succumb to their thyroid cancer. The present study aimed to determine the disease-specific mortality and to identify the risk factors for recurrence.
Material and Methods: This cohort research was conducted in the King Fahad Specialist Hospital in Dammam, Saudi Arabia. The electronic and paper files of all patients with a DTC diagnosis were reviewed. All 460 eligible individuals were risk-stratified using the 2015 American Thyroid Association. The Kaplan-Meier estimator was used to assess the Overall Survival (OS). Results: Out of 460 patients 369 (80.8%) were female. The median age 43.2 years (20 years-85 years). Patients were risk stratified using tumour node and metastasis (TNM) staging as well as American Thyroid Association (ATA) guidelines, based on that 270 (58.6%) patients were low risk, 146 (31.7%) patients intermediate risk and 44 (9.6%) patients fell in the high-risk category. The four patients who died were classified as high risk from the beginning with the following statistically significant risk factors for mortality, the median age of 66 years (p-value= 0.005), N1b lymph nodes metastasis (p-value 0.05) and unstimulated thyroglobulin level >5 (p-value 0.003).
Conclusions: Differentiated thyroid cancer is common, the vast majority are low risk, and with the current strategy of management, the prognosis is excellent. Age > 55 years, presence of distant metastasis, and unstimulated thyroglobulin of >5 were considered factors that negatively influenced the survival.
sushihouse kolstads americanpridefasteners trueforge hotelposeidon youngswoodfinishing arditomason thebestofreno eeclongisland doktervanhecke famsales adla bogatylaw prefplastics kirbycontractinginc unlsp boban dottoressaromolimonica thebestofsanfrancisco vernix digitaldocuments tristatepropertybrokers sotrafib cemcorpny agenziaimmobiliarebuti joesitalianfoodmarket corpoguardiedicitta icsatc antoniniassicurazioni besel strappedincarseatsafety drevobeton blessingconstructionny centre-endorphine qmbtunisia elearning 357 mpress rrappliance ciaautomazioni antikva stubbanvel tacticalpublicrelations mpulshnk paulyboybrand lordshoes ijd-procom thebestrestaurants atkpalvelut ceat leonfukspc pilotexamssa ashgrovecabins universalshielding thebestofcharlotte johnjmazurinc rollnroaster thecabinetwarehouse pocketchangeduo thebestofmilwaukee resan sooli vwe centralwindowcleaning reinforcedplasticslab bugbustersofli ciandrigiardini arfada centrumeigenwijs thebestofmemphis maisonpearly islipll dcgraphicsinc agetranquille relisandroth thebestofcharleston traiteur-wn safi-ingenierie justmyvoice harrishardware louisbarbatolandscaping brightstartoursworld rga-insurance calacatajumper ezantia mrcheapocds van4holiday cigap richtour fagyhatar sicc weshopmall ventovuori sobatrapcapbon unityrubberllc thebestofsaltlakecity ircinc sescoindustries connply storen-servicesenter psnry greatneckcollision ironfitendurance tuscanycountryhouse sansovinocalcio bbradydesign samsam vdtarification springersoil thebestofdayton milantechnology labradoodlesoflongisland daliamohamed theconsultantpowerhouse gunnbrush kruunuosk shulmanproduce balcosupply conso-med federalnetworks bellmoreglass alwaysaffordableconcrete jayteeinsurance carolina-cabins cmorfinance stretchritepackaging dishaairwaysenterprise biocontrol distribio peterivill lcc inspekta jvidesigns royalroseinc calabriapizza fadhila prato-pronto-effetto arbemachine olsonelectricnj tkbl tuttifotokft alpernmd ggsupplywholesale almaxcorporation sotuflex ecustomgutter studenipotok metal-lineconcept rettsodontologi nutecsystems aclotbeach gjonnes-bygg cavalierinternational mnemos southfloridashuttles ajchemicalsupply rachelsfireisland concepto nesponge ultimatestylesofamerica techniquesmadeeasydrivingschool stevesmeatsfreeport eastwest gritbrush plussplan biosens viltkam wikaya paintballconcept justmyvoice securecarkeysupply justmyvoice allcountylegal catt plugandcharge ttandlcontracting justmyvoice islandboatlettering gms-tunisie sportsiena bloeiop touchofclasscollision kotekservice ittoscana pallongislandlacrosse industrialfinishings painoutband rakvag-batforening konemies rrfamilychiropractic infienile shbcgroup footpharmacydirect scuolaguidaprato ans-nettoyage autoskola saafa autolaky1 fixcars longislandelitelandscaping rayscan palaconstruction studio44 crealhome viniferi gavinburke ciprianigiardini davidpokorny osteriailcapodaglio thebestoffairfax centroorafofaccioli ateliervb bbdps thebestoffresno prodigus stratpak umisushirestaurant sotim suldalrenovasjon amer-equip k-kleven jerryspridepotatoes neuroky psicologozampoli jjslandscaping thebestofoklahomacity responsivesales paratie-antiallagamento-shop hearproof cfat ruspinameubles planetbioplastics lynbrook-plumber fcdf-ye brechanparkett valley-stream-plumber diagnosismaker aquavaria jedit garageennour heimdalbygg thebestoflittlerock raybomarine bayshorepaper gmstowing potatura-abbattimento-piante thebestoflouisville michaelalbert thebestofannarbor unitypavers mtnfueloil durub-mudiya lesgensdere dormerking thusney italianvistatravel maiemad gtiuniformcleaning suddenimpactli levituuli sourisalavie mayoiltank mschwartzfeather rands arieslimousines orthoticworld longislandcocktailhours waterjet allislandpaving dukediagnostic klfgoteborg yanezviaggi apruk decogato springeroilltd semapsolar irisgioiellicomprooro msedpsoftware fourcmanagement holemans 7consulting medinet evertile robertwitcomblandscape ceramics adrobotengineering volt-energy huisjacobs chimneyserviceboston eastmainstdental gcbt rememberingbriank elligiardiniespurghi paulslandscaping luisrestorations mgstunisie fixcarsny crcdd hotelprincipessalucca turvahallinta ralphjr thales irrigazione-giardini spantecsystems biomedic fgt-trading sols-egypt spectrumlaboratoriesinc prosecurite spongewarehouse afsainc dellafrancadevelopmentgroup leragazzedifirenze ferrettiwatches dovreentreprenor digitalhvac mostlymica fleurs-velghe autoscuolalebadie interlockingrubbertiles meadowcreekhoa bcn corpjetsupport jrkitchensflooring arteletti
Keywords
differentiated thyroid cancer, malignancy, survival, unstimulated thyroglobulin distant metastasis.
Introduction
Differentiated Thyroid Carcinoma (DTC) developing from thyroid follicular cells encompasses both papillary and follicular histological subtypes, which contribute to more than 90% of all thyroid malignancies. Although the majority of patients with DTC diagnosis have long-term survival, a considerable number may have a persistent or recurrent illness, and some may ultimately succumb to their thyroid cancer. An accurate prognosis is required to determine whether patients may benefit from more or less aggressive treatment, but existing staging approaches still fail to account for a substantial proportion of the variability in illness outcomes [1,2]. Notwithstanding, incidence rates are on the rise, and the demand for precise risk classification and longterm outcome data to support treatment choices is growing for patients with early-stage, extremely low-risk malignancies as well as those with advanced and metastatic illness.
DTC is the second most frequent cancer in middle-aged Saudi Arabian women [3]. Children (age 18 or under), teenagers (age 25 and under), and young adults (age 25 and under 30) contribute to 3%-10% of DTC cases, which is quite rare. According to recent studies, pre-puberty, puberty, and adolescent development periods affect DTC frequency in children differently [4, 5].
Standard therapies for thyroid cancer consist of surgery, Radioactive Iodine-131 (RAI), and Thyroid Hormone Suppression Medication (THST). In the absence of prospective studies, there is much disagreement over the ideal length of surgery and the efficacy and dosage of postoperative RAI. Consequently, retrospective studies with low occurrence rates and/or expert opinion have been heavily relied upon [6]. Identifying potentially aggressive disease cases in advance and evaluating the most appropriate treatment strategy for each case has been a challenge. The present study aimed to determine the disease-specific mortality and to identify the risk factors for recurrence.
Material and Methods
Study Design and Population:
This cohort research was conducted in the King Fahad Specialist Hospital in Dammam, Saudi Arabia. Patients who registered in the hospital's database between January 1, 2011, and December 31, 2018, constituted the research population. Histologically verified patients of thyroid carcinoma according to the International Classification of Diseases for Oncology, Third Revision, site codes (C73.0- 73.9), who followed a therapy plan and followup at KFSHD, were deemed eligible. We included patients who were Saudi and at least 18 years old but excluded those who were younger. If their radioactive iodine administration was planned outside of KFSHD and patients with a prior history of additional primary tumours or thyroid carcinoma were also excluded[7,8].
The KFSHD Research Ethics Board Committee approved the research. The electronic and paper files of all patients with a DTC diagnosis were reviewed. All 460 eligible individuals were riskstratified using the 2015 American Thyroid Association (ATA) standards [9].
Socio demographic variables (gender and age at diagnosis), tumour characteristics (tumour size, stage, histological type, regional lymph node metastasis, and distant metastasis), and treatment variables (type of surgery, unilateral or bilateral, cervical and/or mediastinal lymphadenectomy, radioactive iodine, and external beam radiotherapy) were investigated. Based on the 7th and 8th editions of the TNM staging method, pathological staging (pTNM) was used [10, 11]. One of the variations between the seventh and eighth editions of TNM is the increase from 45 years to 55 years for the age of poor prognosis. In addition, the T3 definition has been updated, and there have been modifications to age categories for those less than 55 years old. Because the eighth edition of TNM staging was released on January 1, 2017, we applied it to patients seen after that date. The outcome variable was the interval between illness diagnosis and recurrence or death. When the patient had been receiving therapy for at least a year, we documented the treatment's outcome in the most recent followup appointment. We tracked the recurrence period for patients who had it, and we performed a multivariate analysis to determine the risk variables. To determine the risk variables for mortality, we performed the same study on individuals who had passed away. The following definitions of response to treatment were employed, which are taken from the ATA 2015 guidelines:
Excellent response no clinical, biochemical (unstimulated thyroglobulin <0.2 ng/mL), or structural evidence of disease.
Biochemical incomplete response: abnormal Thyroglobulin (Tg)(unstimulated >0.1 or stimulated level >10 ng/mL) or rising antiTg antibody levels in the absence of localizable disease.
Structural incomplete response: persistent or newly identified loco-regional or distant metastases.
Indeterminate response: nonspecific biochemical or structural findings that cannot be confidently classified as either benign or malignant. This includes patients with stable or declining anti-Tg antibody levels without definitive structural evidence of disease and patients with unstimulated TG <0.1 or stimulated level <10 ng/mL.
We defined recurrence as evidence of the disease with raising Tg or anti-Tg antibody and/ or new abnormal findings in imaging studies with confirmed malignant tissue by Fine Needle Aspiration (FNA) if needed.
Statistical analysis:
For all continuous variables, such as age, descriptive findings are provided as mean Standard Deviation (SD) or median with range (for data not normally distributed), while all categorical variables are represented as numbers (%). (e.g.,gender). When necessary, bivariate analysis was done using the independent sample t-test, Mann Whitney U-test, Pearson Chi-Square, and Fisher Exact test to determine the association between each demographic factor and each clinical factor and mortality. The date of operation served as the starting point for the time-to-event analysis. The KaplanMeier estimator was used to assess the Overall Survival (OS). To find important variables linked to Overall Survival, a Cox proportional hazard model was applied (OS). Both the hazard ratio and its 95% confidence interval were provided. Statistical significance was defined as a p-value of 0.05.
Results
Out of 460 patients, 80 were men (19.1%) and 369 were women (80.8%). Those who were diagnosed on average were 44 years old (median age 43.2 years, ranging from 20 year -85 years). Based on TNM staging and ATA standards, 270 (58.6%) patients were deemed to be at low risk, 146 (31.7%) patients were deemed to be at intermediate risk, and 44 (9.6%) patients were deemed to be at high risk (Table 1). Among the patients treated, 365 (82.2%) had an excellent response, 19 (9.0%) had incomplete biochemical response, 32 (7.2%) had incomplete structural response, and 27 (6.1%) had an indeterminate response (Figure 1). The median follow up was 5 years (3 years to 7 years). 17 patients (3.6% of the total) had a recurrence. Kaplan-Meier plot (Figure 2) shows that out of 460 patients, 99.1% survived. Four patients succumbed to their illness, with a disease-specific death rate of 0.9% (Figure 3).
Tab. 1. Socio-demographic and clinical characteristics of patients diagnosed with Differentiated thyroid cancer by histological type
| Variables | N | % | |
|---|---|---|---|
| Gender | Female | 369 | 80.8 |
| Male | 80 | 19.1 | |
| Age | <55 | 365 | 79.3 |
| >55 | 95 | 20.7 | |
| Histology type | PTC | 402 | 87.4 |
| FTC | 58 | 12.6 | |
| Size | >1 cm | 115 | 25 |
| 1-4 cm | 255 | 55.4 | |
| >4 cm | 90 | 19 | |
| Regional lymph node metastasis | No | 320 | 69.6 |
| Yes | 140 | 30.4 | |
| Distant metastasis | No | 435 | 94.6 |
| Yes | 25 | 5.5 | |
| Risk stratification | Low risk | 270 | 58.6 |
| Intermediate risk | 146 | 31.7 | |
| High risk | 44 | 9.6 | |
| Total thyroidectomy | 354 | 80.1 | |
| Lobectomy | 82 | 18.6 | |
| Radioactive iodine therapy | 313 | 70.8 | |
| Number of recurrences | 17 | 3.6 | |
| Deaths | 4 | 0.9 |
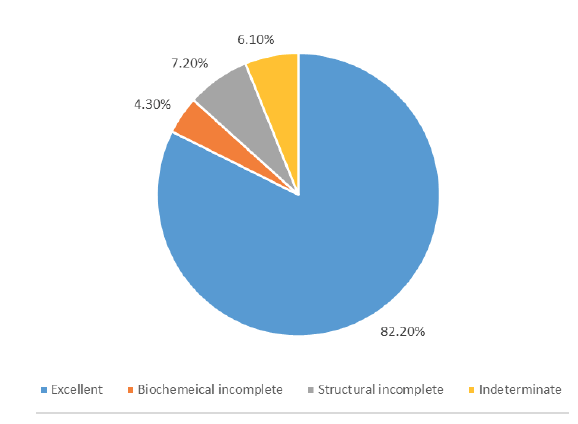
Figure 1: Response to therapy
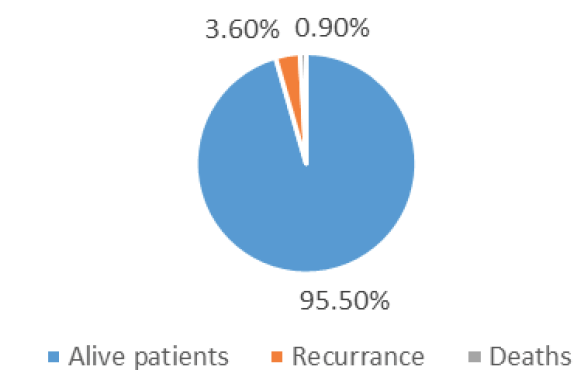
Figure 2: Risk of recurrence and mortality
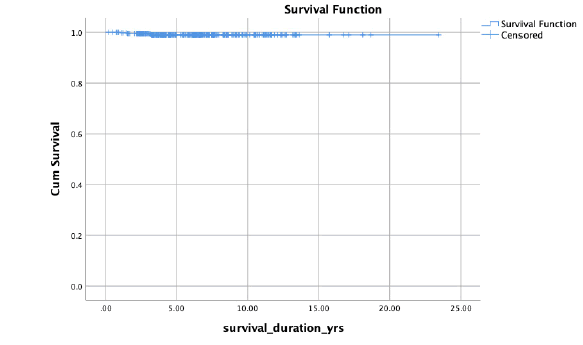
Figure 3: Kaplan-Meier plot of disease specific survival in differentiated thyroid cancer patients.
Table 2 displays the risk variables for death for the four patients who passed away: median age of 66 years (p-value=0.005), metastases to b1 lymph nodes (p-value = 0.05), and unstimulated thyroglobulin level >5 (p-value = 0.003). Lung, brain, and bone metastases were found in 3/4 of individuals (P - 0.017) (Table 2). Regarding recurrence, the average period between diagnosis and recurrence was 57.4 months (min 1-max 131). Initial risk categorization for each patient was high or intermediate (p-0.001). Patients who had an incomplete structural 6/17 or incomplete biochemical response after radioactive iodine therapy 15/17 (p-value=0.001), the presence of residual tissue postoperatively (p-value= 0.05), and the presence of extra thyroid extension (p-value=0.002) are all risk factors for recurrence (Table 3) [6]
Tab. 2. Risk factors for mortality
| Risk factor | P-value |
|---|---|
| Median age 66 | 0.005 |
| Unstimulated TG >5 | 0.003 |
| Presence of distant metastasis | 0.005 |
Tab. 3. Risk factors for recurrence
| Risk factor | P-value |
|---|---|
| Number of metastatic lymph-nodes >3 | 0.002 |
| Presence of any extra thyroid extension | 0.014 |
| Presence of residual thyroid tissue post operatively | 0.05 |
| Incomplete structural response post iodine therapy | 0.001 |
| Incomplete biochemical response post iodine therapy | 0.001 |
| Presence of distant metastasis | 0.001 |
Discussion
For DTC, survival analysis is particularly difficult because of the low disease-related mortality rate. In this study, the probability of dying because of DTC was 0.9 % in 5 years. Mazzaferi et al. [11] observed death probability at 4.0%, 6.0%, and 8.0% at 10 years, 20 years, and 30 years, respectively, in a cohort of patients with DTC in the United States. The lower rate we observed in our study is possibly due to shorter follow-ups and a smaller number of patients. As expected, high-risk individuals as per ATA guidelines had much lower survival.
The present study reported that three out of four dead patients had distant metastasis to the lungs brain and bones (p-value=0.017). It have described the main predictive factors affecting survival; the presence of distant metastases, large tumour size, and lymph node involvement significantly predicted a poor outcome [11, 12].
The present study reported that the median age of dead patients was 66 years. Kelly et al. reported that age is a significant factor, especially for age over 55 years old [13]. Indeed, age above 45 years old was correlated with a reduced survival rate as suggested by many other studies, including the latest ATA references [14-16]. With the new staging AJCC/TNM system, the age cut-off was changed to 55 years [17, 18].
The recurrence rate in our study is 3.6% and the median time to recurrence in our patients was 4.75 years. There is a wide range of recurrence rates in the literature that can reach up to 20 % during the patient's lifetime depending on the risk status [19]. The average time to recurrence has been reported in the literature anywhere from 6 months to decades later [20, 21]. Our low recurrence rate is possible because of the short duration of follow-up. Predictors of recurrence are as expected.
Conclusion
Differentiated thyroid cancer is common, the vast majority are low risk and with the current strategy of management, the prognosis is excellent. Age >55 years, presence of distant metastasis, and unstimulated thyroglobulin of >5 were considered factors that negatively influenced the survival.
Acknowledgment
The author is thankful to all the associated personnel, who contributed to this study by any means.
Data Availability
The data will be available for review from the corresponding author upon request.
References
- Kilfoy BA, Zheng T, Holford TR, Han X, Ward MH,et al. International patterns and trends in thyroid cancer incidence, 1973–2002. Cancer causes control 2009 ;20:525-531.
- Elisei R, Molinaro E, Agate L, Bottici V, Masserini L,et al. Are the clinical and pathological features of differentiated thyroid carcinoma really changed over the last 35 years? Study on 4187 patients from a single Italian institution to answer this question. J. Clin. Endocrinol. Metab. 2010 ;95:1516-1527.
- Hussain F, Iqbal S, Mehmood A, Bazarbashi S, ElHassan T, et al. Incidence of thyroid cancer in the Kingdom of Saudi Arabia, 2000–2010. Hematol Oncol Stem Cell Ther 2013; 6:58–64.
- Jarzab B, Handkiewicz-Junak D. Differentiated thyroid cancer in children and adults: same or distinct disease? Hormones (Athens). 2007; 6:200-209.
- Kim SS, Kim SJ, Kim IJ, Kim BH, Jeon YK,et al. Comparison of clinical outcomes in differentiated thyroid carcinoma between children and young adult patients. Clin. Nucl. Med. 2012;37:850-853.
- Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL,et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association (ATA) guidelines taskforce on thyroid nodules and differentiated thyroid cancer. Thyroid. 2009 ;19:1167-1214.
- Mazzaferri EL, Kloos RT. Current approaches to primary therapy for papillary and follicular thyroid cancer. J. Clin. Endocrinol. Metab. 2001;86:1447-63.
- McGriff NJ, Csako G, Gourgiotis L, Guthrie LC, Pucino F,et al. Effects of thyroid hormone suppression therapy on adverse clinical outcomes in thyroid cancer. Ann. med. 2002 ;34:554-564.
- Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE,et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016 ;26:1-33.
- Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM classification of malignant tumours. John Wiley Sons; 2011.
- Nixon IJ, Wang LY, Migliacci JC, Eskander A, Campbell MJ,et al. An international multi-institutional validation of age 55 years as a cutoff for risk stratification in the AJCC/UICC staging system for well-differentiated thyroid cancer.Thyroid. 2016;26:373-380.
- Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medicalbtherapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418-428.
- Podnos YD, Smith D, Wagman LD, Ellenhorn JD. The implication of lymph node metastasis on survival in patients with well-differentiated thyroid cancer. The American Surgeon. 2005;71:731-744.
- Zaydfudim V, Feurer ID, Griffin MR, Phay JE. The impact of lymph node involvement on survival in patients with papillary and follicular thyroid carcinoma. Surgery 2008 Dec;144:1070-1078.
- Kelly A, Barres B, Kwiatkowski F, Batisse-Lignier M, Aubert B,et al. Age, thyroglobulin levels and ATA risk stratification predict 10-year survival rate of differentiated thyroid cancer patients. PloS one 2019;14.
- Haugen BR, Sawka AM, Alexander EK, Bible KC, Caturegli P,et al. American Thyroid Association guidelines on the management of thyroid nodules and differentiated thyroid cancer task force review and recommendation on the proposed renaming of encapsulated follicular variant papillary thyroid carcinoma without invasion to noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Thyroid 2017;27:481-483.
- Schvartz C, Bonnetain F, Dabakuyo S, Gauthier M, Cueff A,et al. Impact on overall survival of radioactive iodine in low-risk differentiated thyroid cancer patients. The J. Clin. Endocrinol 2012 ;97:1526-1535.
- Jonklaas J, Nogueras-Gonzalez G, Munsell M, Litofsky D, Ain KB,et al. The impact of age and gender on papillary thyroid cancer survival. J Clin Endocrinol Metab. 2012;97:878–887.
- Tuttle RM, Haugen B, Perrier ND. Updated American Joint Committee on cancer/tumor-node-metastasis staging system for differentiated and anaplastic thyroid cancer: what changed and why?. Thyroid. 2017;27:751-756.
- Grogan RH, Kaplan SP, Cao H, Weiss RE, DeGroot LJ, et al. A study of recurrence and death from papillary thyroid cancer with 27 years of median follow-up. Surgery. 2013; 154:1436-1447.
- Brassard M, Borget I, Edet-Sanson A, Giraudet AL, Mundler O,et al. Long-term follow-up of patients with papillary and follicular thyroid cancer: a prospective study on 715 patients. J Clin Endocrinol Metab. 2011; 96:1352-1359.



